Abstract
We present a case of cytokeratin (CK) 20-positive large cell neuroendocrine carcinoma (LCNEC) presenting with multiple skin metastases as the primary manifestation. The patient was a 55-year-old man who presented with a one- month history of subcutaneous skin colored nodules of various sizes on his trunk. Pathologic examination of the skin revealed a nested and solid proliferation of large undifferentiated cells with vesicular nuclei and prominent nucleoli. Tumor cells were found to be immunohistochemically positive for CK 20, chromogranin A, synaptophysin, and CD56. Based on these features, the tumor was diagnosed as a large cell neuroendocrine carcinoma with multiple skin metastases. Computed tomographic (CT) imaging found metastatic foci in the liver, pleura, bone, and lymph nodes. We were unable to identify the primary site of origin. To the best of our knowledge, this is the first case of a large cell neuroendocrine carcinoma with a primary manifestation of multiple skin metastases.
Neuroendocrine tumors (NET) are characterized by features of both neuroendocrine and epithelial differentiation based on immunohistochemical and biochemical examinations. Specific types of NETs include small cell carcinoma (SCC), typical and atypical carcinoid tumors, and large cell neuroendocrine carcinoma (LCNEC).
LCNEC of the lungs was first described by Travis et al.1 LCNEC presents with typical features of NET, including organoid nesting, palisading, rosettes, trabecular patterns, high mitotic rate, and positivity for neuroendocrine markers based on immunohistochemistry. Occurrence of LCNEC is rare; however, the histological and immunohistochemical profile, as well as the prognosis for these tumors, are not well defined.
In a study of metastatic skin tumors, Chu and associates2 showed cytokeratin (CK) 20 positivity in almost all colorectal carcinomas and Merkel cell tumors, pancreatic carcinomas (62%), gastric carcinomas (50%), cholangiocarcinomas (43%), and transitional cell carcinomas (29%), and was nearly absent in carcinomas from other organ systems. However, in our case, LCNEC was found to be immunohistochemically positive for CK20, regardless of the original primary site. Expression of CK20 may not be useful in determining the site of origin for patients with LCNEC.
A 55-year-old man presented with a one-month history of skin lesions on his trunk. The lesions were well-circumscribed, skin-colored, asymptomatic subcutaneous nodules measuring 1~2 cm (Fig. 1). Several new lesions had developed on the head, neck, and leg within the past 10 days. Tumor marker values revealed an increase in serum carcinoembryonic antigen (CEA) (CEA; 513 ng/ml, normal range <4.1 ng/ml), carbohydrate antigen (CA) 19-9 (CA 19-9; 8,457 ng/ml, normal range <37 U/ml), and alpha fetoprotein (aFP; 37.13 ng/ml, normal range <4.0 ng/ml).
The patient had been a chronic hepatitis B virus (HBV) carrier for the previous five years. However, previous liver enzyme levels and abdominal computed tomographic (CT) images were unremarkable. Histopathologic examination of a subcutaneous nodule on the trunk showed cohesive sheets of cells with a prominent trabecular or organoid arrangement. The tumor was primarily located in the subcutaneous fat layer without connections to the epidermis and dermis. It showed nests composed of pleomorphic cells with surrounding lymphoid stroma (Fig. 2). Tumor cells were pleomorphic and larger than those seen in conventional Merkel cell carcinoma. Nuclei of tumor cells were hyperchromatic and vesicular with prominent nucleoli (Fig. 2). Mitotic figures were prominent. Cells showed positive immunoreactivity for CD56, chromogranin, and synaptophysin. CK20 expression was diffuse in the cytoplasm; however, no dot-like perinuclear positivity was observed (Fig. 3). Tumor cells showed negative immunoreactivity for HMB45, S-100, CK7, LCA, and TTF-1 (Thyroid transcription factor-1), vimentin, and neurofilament.
CT imaging showed enhanced masses on the liver, pleura, bone, and lymph nodes. The colon and rectum were found to be intact by colonoscopy. However, we were not able to identify the primary site of the malignancy. Diagnosis of a large cell neuroendocrine carcinoma with multiple skin metastases was established, and the patient received chemotherapy based on cisplatin and etoposide. Two months after appearance of cutaneous lesions, the patient died from septic shock.
LCNEC is characterized by (I) neuroendocrine appearance on a light microscope, (II) large cell size (up to three times larger than normal lymphocytes), polygonal shape, low nuclear-cytoplasmic ratio, coarse nuclear chromatin and frequent nucleoli, (III) high mitotic rate (>10/10 high-power fields (HPF)) and frequent necrosis, and (IV) neuroendocrine features detected by immunohistochemistry or electron microscopy1.
LCNEC has recently been reported in various organs, including the lungs3, breasts4, uterine cervix5, gallbladder6, urinary bladder7, ovaries8, and colon9. Distant metastases from a primary visceral LCNEC tumor are not unusual. Liver and bone were the most common sites of metastases4. Cutaneous metastasis from neuroendocrine carcinomas of visceral origin has rarely been described. Only two cases of LCNEC presenting with skin metastasis have been reported in the literature10,11. The primary sites of origin were the urinary bladder and rectum, and both tumors appeared as a single lesion on the scalp. In fact, no cases of multiple cutaneous metastasis, such as this case in LCNEC, have been reported. In particular, a case of cutaneous metastasis originating from the urinary bladder showed extremely poor prognosis, and, like this case, the patient died after two months due to cutaneous metastasis9.
The distribution of CK20, a 46 kDa basic protein, is almost entirely confined to the gastrointestinal epithelium, urothelium, and Merkel cells6. Colorectal adenocarcinomas strongly express CK20 in more than half of the tumor cells2,12. Due to unremarkable findings on colonoscopy, we excluded the colon as the primary site of malignancy. Thyroid transcription factor-1 (TTF-1) is a 38 kDa transcription factor expressed in the thyroid, lungs, urinary bladder, and certain areas of the brain10,13. In this case, a stain for TTF-1 was negative and there was no evidence of invasion into the urinary tract. Cutaneous metastatic NEC must be differentiated from Merkel cell carcinoma. In addition, its in differentiation between LCNEC and Merkel cell carcinoma when predicting the prognosis of a patient. LCNEC is distinguished from Merkel cell carcinoma by the presence of larger and more pleomorphic cells, which have prominent nucleoli and lack dot-like positive staining for CK20. In this case, cytoplasmic staining for CK20 was found, and cells seemed larger and more pleomorphic, this case was considered as LCNEC.
Clinically, cutaneous involvement of Merkel cell carcinoma is seen primarily in areas of the skin that have high sun exposure, including the head, neck, and extremities. Less than ten percent of cases of Merkel cell carcinoma are reported on the trunk. Most patients with Merkel cell carcinoma are in their seventh decade or older. Only five percent are below the age of 50 at the time of diagnosis14. In the present case, based on histopathology, the tumor mass was limited to the subcutis. Tumors were first seen on the trunk in a relatively young patient and the progression was very rapid. Due to the sudden onset and appearance of multiple subcutaneous lesions, we were strongly suspicious of metastatic carcinoma from other visceral organs. Therefore, we presumed that the lesions were cutaneous metastases from visceral LCNEC.
Nassar et al.15 reported that CK20 expression was seen in one third of the high grade neuroendocrine carcinomas of the ampulla of Vater; however, it was not in any small cell carcinomas. Adegbola et al.16 reported that small cell NEC of the breast showed focal positivity for CK7 and was negative for CK20. Seven cases of CK20 positive LCNEC have been reported4,10,11,17-19. Primary sites of origin included the colon, rectum, ovaries, skin, urinary bladder, and breasts (Table 1). CK20 shows a membranous or cytoplasmic staining pattern with focal or diffuse positivity. Four cases showed a weak and focal pattern and one case showed a diffuse pattern similar to that of the present case. Therefore, positivity for CK20 is thought to be seen in LCNEC. Further studies are necessary in order to understand the implications of CK20 positivity in patients who have LCNEC.
We have described the first case of multiple cutaneous metastases from an LCNEC of unknown origin. LCNEC is very rare, and its biological behavior and immunohistochemistry have not been well documented.
Distinguishing between Merkel cell carcinoma and LCNEC showing positive staining for CK20 on the skin lesion is necessary.
Figures and Tables
Fig. 1
Skin examination revealed multiple well-demarcated, skin-colored subcutaneous nodules on the trunk.
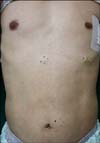
Fig. 2
Microscopic findings of a large cell neuroendocrine carcinoma. (A) Low-power view showing sheets of tumor cells with a trabecular or organoid growth pattern in the subcutaneous area. (B) High-power view showing large pleomorphic cells with hyperchromatic nuclei, coarse chromatin, and numerous mitotic figures (H&E, A: ×40, B: ×200, respectively).

References
1. Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB Jr, Nieman L, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991. 15:529–553.

2. Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000. 13:962–972.

3. Jiang SX, Kameya T, Shoji M, Dobashi Y, Shinada J, Yoshimura H. Large cell neuroendocrine carcinoma of the lung: a histologic and immunohistochemical study of 22 cases. Am J Surg Pathol. 1998. 22:526–537.
4. Okoshi K, Saiga T, Hisamori S, Iwaisako K, Kobayashi H, Ogawa H. A case of cytokeratin 20-positive large-cell neuroendocrine carcinoma of the breast. Breast Cancer. 2009. [Epub ahead of print].

5. Wang KL, Yang YC, Wang TY, Chen JR, Chen TC, Chen HS, et al. Neuroendocrine carcinoma of the uterine cervix: a clinicopathologic retrospective study of 31 cases with prognostic implications. J Chemother. 2006. 18:209–216.

6. Sato K, Waseda R, Tatsuzawa Y, Fujinaga H, Wakabayashi T, Ueda Y, et al. Composite large cell neuroendocrine carcinoma and adenocarcinoma of the common bile duct. J Clin Pathol. 2006. 59:105–107.

7. Dundr P, Pesl M, Povýsil C, Vítková I, Dvorácek J. Large cell neuroendocrine carcinoma of the urinary bladder with lymphoepithelioma-like features. Pathol Res Pract. 2003. 199:559–563.

8. Veras E, Deavers MT, Silva EG, Malpica A. Ovarian nonsmall cell neuroendocrine carcinoma: a clinicopathologic and immunohistochemical study of 11 cases. Am J Surg Pathol. 2007. 31:774–782.
9. Grabowski P, Schönfelder J, Ahnert-Hilger G, Foss HD, Heine B, Schindler I, et al. Expression of neuroendocrine markers: a signature of human undifferentiated carcinoma of the colon and rectum. Virchows Arch. 2002. 441:256–263.

10. Lee WJ, Kim CH, Chang SE, Lee MW, Choi JH, Moon KC, et al. Cutaneous metastasis from large-cell neuroendocrine carcinoma of the urinary bladder expressing CK20 and TTF-1. Am J Dermatopathol. 2009. 31:166–169.

11. Lee WJ, Oh SH, Chang SE, Lee MW, Choi JH, Moon KC, et al. Skin metastasis of neuroendocrine carcinoma arising in the rectum. Ann Dermatol. 2007. 19:163–165.

12. Miettinen M. Keratin 20: immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol. 1995. 8:384–388.
13. Ordóñez NG. Thyroid transcription factor-1 is a marker of lung and thyroid carcinomas. Adv Anat Pathol. 2000. 7:123–127.

14. Pulitzer MP, Amin BD, Busam KJ. Merkel cell carcinoma: review. Adv Anat Pathol. 2009. 16:135–144.
15. Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 2005. 29:588–594.
16. Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005. 58:775–778.

17. Kato T, Terashima T, Tomida S, Yamaguchi T, Kawamura H, Kimura N, et al. Cytokeratin 20-positive large cell neuroendocrine carcinoma of the colon. Pathol Int. 2005. 55:524–529.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download