Abstract
Syringocystadenoma papilliferum (SCAP) and tubular apocrine adenoma (TAA) are rare benign sweat gland tumors. SCAP and TAA may have a histopathologic overlap, but few cases of a SCAP combined with a TAA have been reported. Herein we describe an unusual case of a SCAP co-existing with a TAA located on the back of a 12-year-old girl.
Syringocystadenoma papilliferum (SCAP) and tubular apocrine adenoma (TAA) are rare, benign, sweat gland tumors. In some cases, TAAs can arise in association with SCAPs, and SCAPs are often situated in the superficial portion of TAAs1-11. In a few cases of TAAs associated with SCAPs, pre-existing sebaceous nevi have been described1-6,8,9. Herein, we describe an unusual case of a SCAP accompanied by a TAA without a sebaceous nevus on the back of a 12-year-old girl.
A 12-year-old girl presented with multiple confluent erythematous papules forming a eroded plaque on the left lower back that had been present since birth. The lesion slowly enlarged with time. The patient had no other significant skin lesions, and the medical and family histories were unremarkable. On cutaneous examination, a 1.8×2.5 cm, well-defined, erythematous eroded plaque was situated on the left side of the lower back with a papillomatous surface and a rubbery consistency (Fig. 1). An excisional biopsy was performed (Fig. 2A). On the histopathologic examination, the overlying epidermis showed hyperkeratosis, acanthosis, and papillomatosis. The upper part of the lesion showed cystic and irregularly dilated tubular structures with deep invaginations, from which emerged thick papillomatous projections lined with two rows of epithelial cells. The luminal raw of cells consisted of columnar cells with oval nuclei and faintly eosinophilic cytoplasm. The outer row of cells consisted of small cuboidal cells with round nuclei and scanty cytoplasm. Plasma cells infiltrated the stroma (Fig. 2B). The deeper part of the specimen exhibited tubular structures of variable sizes. The numerous cystic and branching tubular structures in the dermis were surrounded by a paucicellular fibrous stroma. The peripheral layer of tubular structures consisted of flattened cuboidal cells, and the luminar layer was composed of cuboidal cells. Irregular-shaped tubules have a dual or multilayered epithelium with the peripheral layer representing myoepithelial cells. The cells lining the lumina showed evidence of active decapitation secretion (Fig. 2C). After total excision, no recurrence was noted in the following 4 months.
A SCAP is an uncommon benign sweat gland tumor that is usually first noted at birth or in early childhood and presents as a solitary papule, several papules in a linear arrangement, or as a plaque. The lesion increases in size at puberty, becoming papillomatous and often crusted. A SCAP occurs most commonly on the scalp and face, but in 25% of the cases, it occurs elsewhere (e.g., trunk, upper arms, vulva, and thighs)12. Histopathologically, a SCAP consists of a cystic and papillary growth of epithelial elements projecting downward into the dermis and opening onto the skin surface through one or more orifices. Usually the tumor consists of two layers of epithelial cells: the peripheral layer is cuboidal and the inner, columnar cell layer shows evidence of decapitation secretion. The connective tissue stroma of the papillomatous projections is marked by rich infiltration of inflammatory cells; most notably there is a large number of plasma cells12,13. The origin of the SCAP from either eccrine or apocrine structures has been supported in the literature. It is probable that SCAP arises from undifferentiated cells with the potential to exhibit both apocrine and eccrine modes of epithelial secretion. Most lesions of SCAP appear to be nearly of apocrine origin, but some exhibit eccrine derivation13.
A TAA is also an uncommon sweat gland tumor. TAAs are usually found in middle-aged adults as solitary nodules situated mostly on the scalp. TAAs have numerous, irregular-shaped tubular structures that are usually lined by two layers of epithelial cells. The peripheral layer consists of cuboidal or flattened cells, and the luminal layer is composed of columnar cells13. The tubules are embedded in a paucicellular fibrous stroma11-13. In our case, the superficial portion of the tumor showed typical SCAP characteristics and the lower portion was representative of a TAA.
The association of TAAs with SCAPs was first described by Toribio et al.3 in 1987. Another nine cases of SCAPs combined with TAAs have been reported in the English literature2-10 (Table 1). In these cases, SCAPs were also overlaid on TAAs, as in our case. The lesions of SCAPs vary in morphologic character from smooth and flat to raised and verrucous. They also may present as solitary nodules or plaques, or multiple linear papules14,15. Ten cases of SCAPs combined with TAAs showed a wide variety of surface appearances; the various morphologic characteristics could be associated with a superficial portion of the SCAP.
SCAPs have been found in 8~19% of the lesions of sebaceous nevi, and are one of the most commonly associated appendage tumors of sebaceous nevi13. Six of 10 cases of TAAs associated with SCAPs showed pre-existing sebaceous nevi1-6. Three of 10 cases did not mention sebaceous nevi8-10. In the 3 cases, there might have been no evidence of sebaceous nevi. Only one case of a TAA with a SCAP in the external auditory canal has been reported with no background of sebaceous nevi9. In our case, the lower portion of the lesion with a tubular structure may have been ectopic apocrine glands in an underdeveloped sebaceous nevus because our patient was pre-pubertal. Nevertheless, we concluded that our case was a SCAP combined with a TAA because there was no evidence of a pre-existing sebaceous nevus in the completely excised specimen. The intradermal tubular component may be a part of SCAP16. However, the tubular structures in our case were numerous and consisted of compactly gathered forming islands, and so were characteristic of TAA. Also, our case presented on the back, which is a rare site for a sebaceous nevus to develop17. The present case is the first report of a SCAP accompanied with a TAA on the back without a sebaceous nevus before puberty.
TAAs and SCAPs may exhibit histopathologic overlap. Kazakov et al.13 conducted an interobserver study for a histopathologic reappraisal of tubular adenomas (TAs) and SCAPs with four dermatopathologists. The study confirmed a morphologic overlap between TAs and SCAPs and demonstrated a lack of universally accepted diagnostic criteria to classify the lesions with morphologic overlap between TAs and SCAPs, even among experienced dermatopathologists and pathologists.
Ishiko et al.6 reviewed 19 cases of TAAs described in the literature. In 10 of the 19 TAA cases, the tumor was connected to the overlying epidermis and it was necessary to differentiate the lesion from a SCAP. They described TAAs as being different from SCAPs in several aspects: 1) TAAs show no cystically-dilated apocrine invaginations extending down from the epidermis; 2) papillary projections are absent; and 3) infiltration of plasma cells is rare or absent.
The term tubulopapillary hidradenoma (TPH) was first proposed by Falck and Jordaan18 in 1986, and is a recently proposed category of adnexal tumors that remains controversial. A TPH encompasses a spectrum of lesions from papillary eccrine adenomas (PEAs) to TAAs. This designation indicates a variable degree of differentiation from both apocrine and eccrine glands and it is difficult to precisely distinguish between these types of tumors. A TPH is also closely related to a SCAP. Even though this classification is controversial, it has been proposed that PEAs, TAAs, and SCAPs are an inter-related group of appendage tumors13,19. Recent studies have suggested that these tumors arise from either pleuripotential appendage cells or apo-eccrine glands5.
In summary, we present a case of a SCAP on the lower back that was histopathologically associated with a TAA without a sebaceous nevus. Despite the rare occurrence, this entity can be considered when similar skin lesions are observed.
Figures and Tables
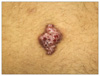 | Fig. 1An erythematous well-defined and papillomatous plaque with superficial erosion and crust on the left side of the lower back. |
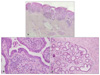 | Fig. 2Views of the tumor. (A) The upper portion of the tumor had a cystic invagination that extends downward from the epidermis, and the lower portion exhibited islands of irregular-shaped tubular structures in the deep dermis at the excision specimen (H&E, ×20). (B) Two different epithelial cell layers lining the invaginated area and papillary projections in the upper portion of tumor. The luminal row consisted of high columnar cells showing active decapitation secretion, and the outer row consisted of small cuboidal cells. There was stromal infiltration of many plasma cells (H&E, ×400). (C) The numerous cystic and branching tubular structures in the dermis surrounded by a paucicellular fibrous stroma. The tubular structures have a dual or multilayered epithelium and the luminal cells showing evidence of active decapitation secretion in the dermis (H&E, ×100). |
References
1. Taylor RS, Perone JB, Kaddu S, Kerl H. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Appendage tumors and hamartomas of the skin. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw Hill;1068–1087.
2. Ahmed TS, Priore JD, Seykora JT. Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Tumors of the epidermal appendages. Lever's histopathology of the skin. 2008. 10th ed. Philadelphia: Lippincott Williams & Wilkins;858–8602.
4. Ansai S, Watanabe S, Aso K. A case of tubular apocrine adenoma with syringocystadenoma papilliferum. J Cutan Pathol. 1989. 16:230–236.

5. Epstein BA, Argenyi ZB, Goldstein G, Whitaker D. An unusual presentation of a congenital benign apocrine hamartoma. J Cutan Pathol. 1990. 17:53–58.

6. Ishiko A, Shimizu H, Inamoto N, Nakmura K. Is tubular apocrine adenoma a distinct clinical entity? Am J Dermatopathol. 1993. 15:482–487.

7. Aktepe F, Demir Y, Dilek FH. Tubular apocrine adenoma in association with syringocystadenoma papilliferum. Dermatol Online J. 2003. 9:7.

8. Ahn BK, Park YK, Kim YC. A case of tubular apocrine adenoma with syringocystadenoma papilliferum arising in nevus sebaceus. J Dermatol. 2004. 31:508–510.

9. Lee CK, Jang KT, Cho YS. Tubular apocrine adenoma with syringocystadenoma papilliferum arising from the external auditory canal. J Laryngol Otol. 2005. 119:1004–1006.

10. Yamane N, Kato N, Yanagi T, Osawa R. Naevus sebaceus on the female breast accompanied with a tubular apocrine adenoma and a syringocystadenoma papilliferum. Br J Dermatol. 2007. 156:1397–1399.

11. Vazmitel M, Michal M, Mukensnabl P, Kazakov DV. Syringocystadenoma papilliferum with sebaceous differentiation in an intradermal tubular apocrine component. Report of a case. Am J Dermatopathol. 2008. 30:51–53.

12. Kim MS, Lee JH, Lee WM, Son SJ. A case of tubular apocrine adenoma with syringocystadenoma papilliferum that developed in a nevus sebaceus. Ann Dermatol. 2010. 22:319–322.

13. Kazakov DV, Bisceglia M, Calonje E, Hantschke M, Kutzner H, Mentzel T, et al. Tubular adenoma and syringocystadenoma papilliferum: a reappraisal of their relationship. An interobserver study of a series, by a panel of dermatopathologists. Am J Dermatopathol. 2007. 29:256–263.

14. Townsend TC, Bowen AR, Nobuhara KK. Syringocystadenoma papilliferum: an unusual cutaneous lesion in a pediatric patient. J Pediatr. 2004. 145:131–133.

15. Hann SK, Choi YS, Choi EH. Syringocystadenoma papilliferum. Ann Dermatol. 1990. 2:100–104.
16. Requena L, Kiryu H, Ackerman AB. Neoplasms with apocrine differentiation. 1998. Philadelphia, PA: Lippicott-Raven.
17. James WD, Berger TG, Elston DM. Epidermal nevi, neoplasms, and cysts. Andrews' diseases of the skin clinical dermatology. 2006. 10th ed. Philadelphia: Elsevier;633–683.
18. Falck VG, Jordaan HF. Papillary eccrine adenoma. A tubulopapillary hidradenoma with eccrine differentiation. Am J Dermatopathol. 1986. 8:64–72.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download