Abstract
Granular cell tumors (GCTs) can be divided into neural type with S-100 reactivity and non-neural type without that. The latter has not been widely recognized and there are only fewer reports available when compared to conventional GCT. A 65-year-old man was presented with the presence of a painless mass on his back. The mass had developed into a small nodule on the scar developed because of previous surgery carried out 2 years ago. The tumor consisted of large, polygonal cells comprising of an enormous number of faintly eosinophilic small granules in the cytoplasm. The cytoplasmic granules were stained positively for periodic acid-Schiff stain. Immunohistochemical stains for S-100 protein and neuron-specific enolase were found to be negative. Herein, we report the appearance of a very rare case of non neural GCT developed on the surgical scar in support with relevant literature reviews.
Granular cell tumors (GCTs) are a wide variety of benign neoplasms, which have characteristic cells with numerous eosinophilic granular cytoplasms as evident by hematoxylin and eosin (H&E) stains. They can be divided into neural type with S-100 reactivity (so-called conventional GCT) and non-neural type without that (so-called non neural GCT). Amongst the two, non neural GCT (NN-GCT) has not been widely recognized although it has rather unique characteristics in terms of morphology and immunophenotype unlike conventional GCT1. Although NN-GCT has been reported additionally since the first description by LeBoit et al.2, NN-GCT originating from scar has not been reported so far; while appearance of conventional GCT on surgical scar has already been introduced3.
Herein, we report a very rare case of development of NN-GCT on the surgical wound site in support with relevant literature reviews.
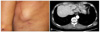
A 65-year-old man was presented to our dermatologic clinic with a painless dermal mass on his back (Fig. 1A). About two years ago, the mass has developed on the latissimus dorsi flap scar and has grown gradually. Latissimus dorsi flap was done for reconstructing skin defect due to the presence of abscess on the patient's left thigh.
Skin examination showed the presence of a non-tender, hard, elevated and well-defined mass on his back measuring 3.5×3.0 cm in dimension.
Computed tomography scan also revealed the presence of a 3.6×2.5 cm sized well-circumscribed oval mass in the subcutaneous layer adjacent to the intercostal muscle and minimal peritumoral fat strands (Fig. 1B).
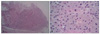
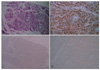
Low power microscopic views revealed the presence of normal epidermis and broad fascicles of tumor cells infiltrating into the deep dermis (Fig. 2A). An increased number of capillaries were present within the lesion. Inflammatory cells were seen focally at the periphery of the tumor. Under high power microscope, the tumor cells were seen as large and polygonal in shape with the presence of numerous, faintly eosinophilic small granules in the cytoplasm (Fig. 2B). The eosinophilic granules were positive for periodic acid-Schiff stains (PAS) and resistant to diastase digestion (Fig. 3A). Immunohistochemical stains revealed positivity for CD68 and vimentin (Fig. 3B). Stains for S-100 protein, neuron-specific enolase (NSE), calretinin, smooth muscle actin and desmin were found to be negative (Fig. 3C, D).
The mass was completely excised and there was no recurrence after 6 months follow-up.
Most of the GCTs are regarded to show neural crest differentiation with positivity for S-100 and these tumors are called as conventional GCT1. However, reports about GCT, which did not express S-100 protein have been continuously published since LeBoit et al. introduced GCTs without S-100 reactivity in 1991 for the first time and these tumors are called as non neural GCT1,4-6. Therefore, NN-GCT is different from conventional GCT in that the latter mostly exhibits neural crest differentiation with S-100 reactivity, but the former does not react with S-100.
NN-GCT is clinically characterized by the presence of painless polypoid or exophytic and well-circumscribed dermal mass, while conventional GCT is usually small and poorly circumscribed4. Primitive NN-GCT can develop at a variety of locations like, the oral cavity, gastrointestinal tract, but skin is the most common site of occurrence. It appears on face, neck, extremities, and trunk5.
No obvious differentiative cell line, which forms NN-GCT has been known so far, despite the presence of a number of studies involving immunohistochemistry1,4,6. It is just postulated that the tumor originates from undifferentiated mesenchymal stem cells6. Therefore, some authors prefer using the terminology, 'primitive non neural granular cell tumor1.
Histologically, epidermal hyperplasia was absent or showed minimal presence in NN-GCT, while pseudoepitheliomatous hyperplasia revealed frequent appearance in conventional GCT4. Lesional tumor cells of NN-GCT are spindle shaped, ovoid or polygonal with abundant eosinophilic granular cytoplasm as revealed by H&E stain; they were also positive for PAS staining and resistant to diastase. Cytologic atypia with a diverse range of mitotic activity, hyperchromatic nuclei and pleomorphism might be demonstrated focally or mildly in NN-GCT1.
The important immunohistochemical feature, which was apparent in NN-GCT was negative expression of S-100 protein, which was not the case in the conventional GCT. Some NN-GCTs were positive for NSE without S-100 protein reactivity, which was not a good evidence for neural differentiation6. Positivity to NIK-C3, a lysosomal antigen and CD 68 were reported, but in association with lack of real cytospecificity. Other mesenchymal markers like, desmin and smooth muscle actin do not react with the tumor cells1.
When GCT occurs secondarily, as in our case, we must be able to differentiate NN-GCT from granular cell modifications developed from pre-existing tumor or granular cell traumatic neuroma.
Granular cell changes have been shown nonspecifically in many other tumors such as, dermatofibroma, rhabdomyoma, basal cell carcinoma, and even occasionally seen in the surgical trauma. However, they involve very limited area of the lesions and do not create a true granular cell tumor1. These changes are much related with the accumulation of the lysosomes, which get increased during cellular degeneration7. Granular cell changes in the surgical trauma are mostly observed near smooth muscle at the sites of previous surgical trauma, especially in the uterine scar8. Granular cell traumatic neuroma could be differentiated from NN-GCT on the basis that the former consists of granular cells and hyperplastic nerve bundles with S-100 reactivity9.
In conclusion, we experienced the appearance of an interesting and rare NN-GCT developed on the previous surgical scar, which has not been reported so far to the best of our knowledge.
Figures and Tables
Fig. 1
(A) Solitary hard, fixed dermal mass on the left lateral chest wall. (B) Computed tomography scan revealing the presence of an oval mass with dimensions of 3.6×2.5 cm in the subcutaneous layer adjacent to intercostal muscle.
References
1. Lazar AJ, Fletcher CD. Primitive nonneural granular cell tumors of skin: clinicopathologic analysis of 13 cases. Am J Surg Pathol. 2005. 29:927–934.
2. LeBoit PE, Barr RJ, Burall S, Metcalf JS, Yen TS, Wick MR. Primitive polypoid granular-cell tumor and other cutaneous granular-cell neoplasms of apparent nonneural origin. Am J Surg Pathol. 1991. 15:48–58.

3. Murcia JM, Idoate M, Laparte C, Baldonado C. Granular cell tumor of vulva on episiotomy scar. Gynecol Oncol. 1994. 53:248–250.

4. Chaudhry IH, Calonje E. Dermal non-neural granular cell tumour (so-called primitive polypoid granular cell tumour): a distinctive entity further delineated in a clinicopathological study of 11 cases. Histopathology. 2005. 47:179–185.

5. Yeh I, Tran DT, Davis TL, Argenyi ZB. An infiltrative variant of non-neural granular cell tumor: a case report. J Cutan Pathol. 2009. 36:Suppl 1. 46–51.

6. Habeeb AA, Salama S. Primitive nonneural granular cell tumor (so-called atypical polypoidgranular cell tumor). Report of 2 cases with immunohistochemical and ultrastructural correlation. Am J Dermatopathol. 2008. 30:156–159.

7. Lee MW, Choi JH, Sung KJ, Moon KC, Koh JK. Granular cell tumor: clinical and histopathological study. Korean J Dermatol. 2000. 38:1030–1036.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download