Abstract
Linear focal elastosis (LFE) is a rare dermal elastosis characterized by hypertrophic yellowish linear plaques and increased abnormal elastic tissues in the lumbosacral area. Although the pathogenesis of this disorder remains unknown, it may be associated with keloidal repair process (KRP) of elastic tissues in striae distensae (SD), because there have been some reported cases of LFE accompanied by SD. We herein report a 14-year-old boy with LFE following SD in the lumbar region. Our case supports the hypothesis of KRP in the pathogenesis of LFE. Immunohistochemical study for transforming growth factor-beta (TGF-β) was negative. Therefore, we assume that the pathogenesis of KRP in LFE is different from that of keloid development, which is the TGF-β signaling pathway.
Since Burket et al.1 described three white elderly men with palpable yellow lines on their back and named the disorder, linear focal elastosis (LFE), in 1989, many cases of LFE have been reported. It usually occurs in the lumbosacral area, characterized by hypertrophic yellowish linear bands and an increase of abnormal elastic tissues2. Although the pathogenesis of LFE remains unknown, it may be associated with keloidal repair process (KRP) of elastic tissue in striae distensae (SD), because some cases of LFE have presented with features of SD3-5. Several cases of LFE, including two cases of LFE with SD, have been reported since we first reported two patients with LFE in the Korean dermatologic literature6-8. We herein report a 14-year-old boy with LFE following SD in the same region. Our case supports the hypothesis of KRP in the pathogenesis of LFE. Additionally, in order to elucidate the role of transforming growth factor-beta (TGF-β) in the pathogenesis of LFE, we performed immunohistochemical study for TGF-β.
A 14-year-old Korean boy presented with a 2-year history of asymptomatic multiple horizontal linear lesions on his lumbar area. His familial and personal histories were unremarkable, and he did not take any significant medications such as steroids. He denied any history of trauma on his lumbar area, obesity, rapid growth, and systemic illness. He recalled that the hypertrophic lesions arose from the atrophic bands, and the atrophic bands on the upper back occurred recently. Physical examination revealed several palpable hypertrophic yellowish bands on the lower lumbar region. The lesions at the middle level of the back showed a transitional change, each of which revealed central hypertrophic bands that joined to peripheral atrophic bands. Above those lesions, slightly atrophic shiny erythematous bands with pinkish hue were seen (Fig. 1). Routine laboratory tests were within normal limits.
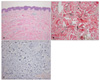
A skin biopsy was taken from a palpable hypertrophic band. On histopathologic examination, hypertrophic collagen bundles were separated by basophilic wavy fibers in the dermis without significant epidermal changes (Fig. 2A). Verhoeff-van Gieson staining revealed irregular clumped or fragmented elastic fibers between collagen bundles in the reticular dermis. Elongated elastic fibers with splitting of ends that looked like a paintbrush were observed (Fig. 2B). Those results confirmed the diagnosis of LFE. In order to elucidate the role of TGF-β in the pathogenesis of LFE, we performed immunohistochemical study for TGF-β, but the result was negative (Fig. 2C).
LFE, or elastotic striae, is a rare dermal elastosis without racial predilection. Patients' ages range from 7 to 89 years, and males are affected more than females2. Clinically, it presents as asymptomatic hypertrophic linear yellow to red plaques that are usually observed incidentally without traumatic history. Although most cases are symmetrically distributed on the lower and middle parts of the back, it may appear on the legs or face9,10. Histopathologically, focal wavy fibers may increase, with separating normal to hypertrophic collagen bundles in the dermis, which are revealed by elastic staining1. The elastic fibers in the upper and deep dermis may be fragmented or aggregated. Electron microscopic examination shows fragmented elastic tissue, microfibrillar or granular components, and aggregated elastin1,11. Elastic microfibrils may be connected with intracytoplasmic filaments of fibroblasts, which suggests that elastogenesis is active11. Although the differential diagnosis of LFE includes various conditions such as SD, pseudoxanthoma elasticum, and dermatofibrosis lenticularis disseminata, they can be easily discriminated from LFE based on the distribution and configuration of the affected lesion2.
The pathogenesis of LFE remains uncertain, but it is linked to alteration of elastic tissues. Although it is somewhat controversial, some authors have suggested that development of LFE might be caused by excessive regenerative changes of elastic fibers from SD, so-called KRP, rather than by de novo synthesis of abnormal elastic tissues4,5. That hypothesis is supported by other authors who described cutaneous findings of LFE contiguous to SD in the same patient and who observed accumulations of thin elastic fibers in the late stage of SD4,12. In particular, Hagari et al.3 reported a 73-year-old woman with central LFE joining to peripheral SD on the back. Two cases of LFE associated with SD were reported in the Korean dermatologic literature. Chang et al.7 reported an 18-year-old healthy boy with LFE on the low back and SD on the knee, and Lim et al.8 reported a 20-year-old man with LFE on the back and SD on the buttocks. While those two cases did not show concurrent presence of skin lesions of LFE and SD in the same region, our case demonstrated coexistence of the two disorders in the same area, with a transitional zone in which the lesion of LFE joined to that of SD. Moreover, the lesions of LFE followed those of SD in our patient. Collectively, our case more clearly supports the hypothesis of KRP, one of the pathogeneses of LFE.
Although the collagen bundles in LFE do not tend to form nodular and whorled masses as in keloid13, LFE exhibits hypertrophic collagen bundles like those in keloid. Sato14 demonstrated over-expression of TGF-β in fibroblasts from keloid tissue by using immunohistochemistry. In order to elucidate whether TGF-β plays a role in KRP and whether it is expressed in fibroblasts in LFE tissue as in keloid, we performed immunohistochemical study for TGF-β, but the result was negative. Therefore, we assume that the pathogenesis of KRP in LFE is different from that of keloid development, the TGF-β signaling pathway.
In summary, we present a case of LFE following SD in a healthy adolescent boy. Our case has more evidence to support the theory that LFE might be caused by regenerative changes from SD. Additionally, we assume that those changes are associated with involvement of various molecules other than TGF-β, although the exact pathway remains unknown. More studies are warranted to find the pathogenesis of LFE and association between LFE and SD.
Figures and Tables
Fig. 1
Multiple horizontally linear palpable mildly yellowish plaques (arrowheads) and several slightly atrophic shiny erythematous striae (arrows) on the middle and lower areas of the back. There are a few bands with a transitional zone (asterisks), in which the central hypertrophic band joined to the peripheral atrophic one in the midst of the back.
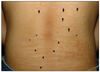
Fig. 2
(A) Hypertrophic collagen bundles were separated by basophilic wavy fibers in the dermis without significant epidermal changes (H&E, ×100). (B) Fragmented (asterisk) or aggregated wavy elastic fibers are present between collagen bundles. Some elastic fibers show a feature resembling a paintbrush (arrow) (Verhoeff-van Gieson stain, ×200). (C) Immunohistochemical study for TGF-β is negative in the dermal fibroblasts, although eccrine sweat glands normally show positive staining (immunoperoxidase stain, ×100).
References
1. Burket JM, Zelickson AS, Padilla RS. Linear focal elastosis (elastotic striae). J Am Acad Dermatol. 1989. 20:633–636.

2. Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: part I. Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004. 51:1–21.

3. Hagari Y, Norimoto M, Mihara M. Linear focal elastosis associated with striae distensae in an elderly woman. Cutis. 1997. 60:246–248.
4. Chang SE, Park IJ, Moon KC, Koh JK. Two cases of linear focal elastosis (elastotic striae). J Dermatol. 1998. 25:395–399.
5. Hashimoto K. Linear focal elastosis: keloidal repair of striae distensae. J Am Acad Dermatol. 1998. 39:309–313.

7. Chang SE, Lee JY, Choi JH, Sung KJ, Moon KC, Koh JK. Linear focal elastosis associated with striae distensae. Korean J Dermatol. 2000. 38:127–129.
8. Lim SH, Ha JH, Kang HA, Park HJ, Baek SC, Kim JW, et al. Linear focal elastosis associated with striae distensae in an atopic patient. Korean J Dermatol. 2001. 39:504–506.
10. Inaloz HS, Kirtak N, Karakok M, Ozgoztasi O. Facial linear focal elastosis: a case report. Int J Dermatol. 2003. 42:558–560.

11. Hagari Y, Mihara M, Morimura T, Shimao S. Linear focal elastosis. An ultrastructural study. Arch Dermatol. 1991. 127:1365–1368.

13. Beer TW, Lam MH, Heenan PJ. Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu G, editors. Tumors of fibrous tissue involving the skin. Lever's histopathology of the skin. 2008. 10th ed. Philadelphia: Lippincott Williams & Wilkin;981–988.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download