Abstract
Giant basal cell carcinoma (BCC), defined as a lesion greater than 5 cm at its largest diameter, is a rare variant of BCC. In contrast to small BCC, giant BCC develops on skin that is not exposed to sunlight, including the back, shoulder, groin and thigh. Most of the histopathologic subtypes of giant BCC are micronodular, morpheaform and nodular, but the superficial subtype is rare. Giant superficial BCC arising on the scalp is extremely rare. We report the case of giant superficial BCC with four satellite lesions on the scalp in a 53-year-old male without predisposing factors.
Basal cell carcinoma (BCC) is the most common human cancer. Most are small and usually occur on sun-exposed areas. The rare variant designated giant BCC has an unusually large size (<5 cm in diameter) and often develops multiple lesions, which frequently occur on skin that is not typically exposed to sunlight, including the back, shoulder, groin and thigh1, 2,3. Patients with giant BCC are more likely to have a tumor that displays an aggressive histologic subtype (morpheaform, micronodular, or metatypical)2. Giant superficial BCCs limited to the scalp are exceedingly rare. We report a case of giant BCC with four satellite lesions on the scalp that was treated with 5% imiqumoid cream and cryosurgery.
A 54-year-old man presented with an asymptomatic darkcolored patch on the frontal scalp. The lesion had been growing very slowly for 15 years, without concern or need for medical attention by the patient. The patient was in good health with no pertinent medical history or concurrent symptoms. There was also nothing contributory from his family history.
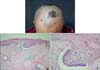
Physical examination revealed a 6×8 cm, well-defined, round black-colored patch on the frontal scalp. On closer examination, four other dark-colored macules were observed around the main lesion (Fig. 1A). Regional lymph nodes were not palpable and the remainder of physical examination was not contributory. Laboratory test results including complete blood cell count, urine analysis, liver function test, chest X-ray and electrocardiogram were within normal limits or negative.
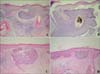
Biopsy specimens were obtained from the center of main lesion and the four satellite lesions. A biopsy specimen from the main lesion showed budding and irregular proliferation of tumor tissue attached to the undersurface of the epidermis. The peripheral cell layer of the tumor formations usually displayed palisading. In addition, a mild amount of a nonspecific chronic inflammatory infiltrate was present in the upper dermis, which is a typical feature for superficial BCC (
Fig. 1B, C). A biopsy specimen taken from the satellite lesions showed similar histological findings (Fig. 2).
The giant BCC was treated a combination of with 5% imiquimod and cryosurgery combination, given the patient's reluctance for an operation and since the lesion was too large to recover by a skin graft. Cryosurgery consisted of 2~3 freeze-thaw cycles (15 seconds of freezing with an intervening 60 second thaw period). Four days later, the patient topically applied 5% imiquimod once. This procedure was done four time. Topical treatment with imiquimod cream was then continued for 4 months. The satellite lesions were addressed by simple excision with a 3 mm margin; free margin resection resulted.
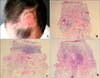
Two months after the last treatment, a clinical examination revealed only atrophic and hypo-pigmented areas, without any signs of residual tumor (
Fig. 3A). Seven months later, follow-up biopsies were performed at three points (center, 6 o'clock and 12 o'clock) at the original site of the giant BCC; the biopsy samples demonstrated only cicatricial fibrosis. At the time of manuscript submission, the patient has been under complete remission for 1 year (Fig. 3B-D).
The American Joint Committee on Cancer Classification of tumors is based on the largest diameter: (T1, ≤2 cm; T2, >2 cm but <5 cm; T3, ≥5 cm). Giant BCC is a T3 tumor2.
Giant BCC presents some common epidemiological factors that include race, multiplicity of tumors, development on sun-covered areas, neglect and tumor chronicity3. Fear may be the underlying factor leading to neglect and chronicity of the tumor2. Immunodeficiency and genetic predisposition to BCC in other family members are not consistent factors3. In contrast to patients who develop a single small BCC, giant BCC frequently develops on skin that is not typically exposed to sunlight, including the back, shoulder, leg and thigh3. The scalp is not a commonly-reported site of giant BCC. Yet, it is conceivable that the presence of scalp hair allows a patient who is fearful of the findings of a medical examination to conceal the tumor4
, allowing the tumor to become chronic and large in size.
Histopathological subtypes of BCC can be grouped as nonaggressive (nodular and superficial subtypes) and aggressive (morpheaform, micronodular and metatypical subtypes)1, 2. In a review of 50 cases of giant BCC, 35 cases (72%) presented an aggressive histopathological subtypes, 13 cases (26%) presented the nodular subtype with only a single case (2%) being the superficial subtype2.
For the treatment of giant BCC, a variety of modalities have been used with inconsistent results. Treatments include surgical excision and grafting, Mohs micrographic surgery or radiation therapy5, with gold standard treatment for large BCC being Mohs surgery. But, surgery carries an increased risk of morbidity since extremely large skin grafts are needed to an area predisposed to hypertrophic scars and keloids6. If patients refuse surgery and skin grafting, alternative treatments must be chosen.
Cryosurgery is widely-accepted for the treatment of well-defined small BCC. Reports have been published demonstrating good results for tumors up to 10 cm7. Recently, giant lesions of superficial BCC were successfully treated by cryosurgery, yielding quite good cosmetic results8. Tissue destruction by cryosurgery starts by the immediate necrosis of treated cells ("physical" phase) and is further strengthened by destruction of the microcirculation of the tumor ("vascular" phase), and as a result of tumor inflammation, probably driven by cryosurgery-triggered liberation of tumor antigens in the organism ("immunological" phase)9, 10.
Similarly, imiquimod has been shown to be effective in the treatment of superficial and nodular BCC with efficacy approaching surgical or ablative modalities. Besides being a potent immune response modifier and stimulator of both innate and adaptive immunity, imiquimod induces apoptosis either by inducing the pro-apoptotic Fas receptor (FasR) or, upon uptake into the cell, by direct intracellular activation of the apoptotic cascade. The result is inhibited angiogenesis by different mechanisms11, 12. Combination therapy of cryosurgery and 5% imiquimod may reinforce apoptosis of the tumor cells, strengthen anti-angiogenesis in the treated tumor and build-up a potent tumor-destructive immune response by a cascade of events starting with imiquimod-promoted attraction of immature dendritic antigen-presenting cells into the tumor. Dentritic cells further mature within the tumor antigen-rich environment of the subsequently cryo- destructed tumor and, upon imiquimod driven migration into the peripheral lymph nodes, can stimulate specific antineoplastic cell-mediated immunity. Finally, continuing imiquimod application after cryosurgery increase recruitment of activated effector cells into the tumor tissue leads to its destruction12.
In summary, we report a case of giant superficial BCC with four satellite lesions on the scalp. Our patient is of interest because of the unusual size, histology and the presence of the satellite lesions. Although further studies are needed to determine the exact causes of this phenomenon, this is the first case of giant superficial BCC with multiple satellite lesions that treated 5% imiqumoid cream and cryosurgery combination therapy.
Figures and Tables
Fig. 1
(A) 6×8 cm sized irregular shaped round pigmented patch with 4 satellite macules on saclp. (B) Tumor shows buds and irregular proliferations of tumor tissue attached to the undersurface of the epidermis (H&E, ×40). (C) The tumor cells have a large, unifrom, oval, nonanaplatic-appearing nucleus with little cytoplasm (H&E, ×100).
References
1. Randle HW. Basal cell carcinoma. Identification and treatment of the high-risk patient. Dermatol Surg. 1996. 22:255–261.

2. Randle HW, Roenigk RK, Brodland DG. Giant basal cell carcinoma (T3). Who is at risk? Cancer. 1993. 72:1624–1630.

3. Sakamoto GK, Izumi AK. . Giant basal cell carcinoma: report of two cases and review of risk factors. Hawaii Med J. 2005. 64:274–276.
4. Lee JH, Park HJ, Kim YC, Cinn YW. A case of giant basal cell carcinoma. Ann Dermatol. 1997. 9:236–238.

5. Rossi R, Campolmi P, Giomi B, Massi D, Cappugi P. Giant exophytic basal cell carcinoma treated with radiotherapy. J Eur Acad Dermatol Venereol. 2002. 16:374–376.

6. Ceilley RI, Del Rosso JQ. Current modalities and new advances in the treatment of basal cell carcinoma. Int J Dermatol. 2006. 45:489–498.

7. Kokoszka A, Scheinfeld N. Evidence-based review of the use of cryosurgery in treatment of basal cell carcinoma. Dermatol Surg. 2003. 29:566–571.

8. Gabbi TV, de Lacerda DA, Maruta CW, de Almeida Pimentel ER. Giant superficial basal cell carcinoma treated with cryosurgery. Dermatol Surg. 2008. 34:1441–1442.
9. Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R. Cryosurgery-a putative approach to molecular-based optimization. Cryobiology. 2004. 48:190–204.

10. Hundeiker M, Sebastian G, Bassukas ID, Ernst KJ, Hölzle E. Cryotherapy in dermatology. J Dtsch Dermatol Ges. 2005. 3:1009–1015.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download