Abstract
Cutaneous γ/δ T-cell lymphoma is an extremely rare and highly aggressive tumor that is often resistant to treatment, such as polychemotherapy and radiotherapy. Due to the complexity of clinical, pathologic, and immunohistochemical features of this disease entity, the physician should perform a careful evaluation; however, treatment should be rapid and aggressive. We present a case of fatal cutaneous γ/δ T-cell lymphoma of a 55-year old woman who died after recurrence with central nerve system metastasis.
Cutaneous gamma/delta (γ/δ) T-cell lymphoma (CGD-TCL) is a rare lymphoma recently defined as a provisional entity according to the latest World Health Organization European Organization for Research and Treatment of Cancer (WHO-EORTC)classification. This lymphoma is composed of mature cytotoxic T cells bearing the γ/δ T-cell receptor. These cells often co-express CD56 and fail to express either CD4 or CD8. It shows a refractory clinical course.
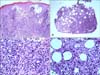
A 56-year-old woman presented at our department with a 6-week history of localized, dusky red colored, centrally umbilicated plaque, with a peripheral erythematous patch on the left foot (Fig. 1). Results from laboratory testing were within normal limits. A first biopsy of the skin lesion revealed epidermal atrophy, dermoepidermal vacuolization, massive cellular infiltration of the entire dermis, as well as a mostly lobular, inflammatory infiltrate of the subcutaneous fat of lymphoid cells and histiocytes (Fig. 2). At high power magnification, the "rimming" of fat cells by pleomorphic, atypical T lymphocytes in the subcutaneous fat was visible (Fig. 2). Following the suspected clinical diagnosis of lymphoma, immunohistochemical stains were performed. Results showed positivity for CD3 (Fig. 3A) and Granzyme B, focally positive for CD30, with high proliferative activity on Ki-67, and negativity for CD4 (Fig. 3B), CD8 (Fig. 3C), CD20, CD56, and βF1 (Fig. 3D). Monoclonal rearrangement of TCRγ genes was detected. All of these findings were consistent with a diagnosis of CGD-TCL. After diagnosis, the patient underwent a total of 4,000 cGy of radiotherapy.
Four months after the first biopsy, the skin lesion recurred on the left foot and a single subcutaneous pea-sized nodule developed on the left axilla. A new biopsy was taken, revealing lymphocytic infiltrates within the superficial and deep dermis, and also the subcutis, with a lobular pattern. Results of immunohistochemical staining were the same as those of the previous biopsy. Based on these findings, we made a diagnosis of recurred and metastasized CGD-TCL, and administered radiotherapy. One month later, the patient underwent magnetic resonance imaging (MRI) for evaluation of pain on the right upper extremities, interscapular area, and radiating pain on the right thigh. MRI showed metastasis (white arrows) in the spinal cord (Fig. 4). The patient died of multiple organ failure with CMV pneumonia and pancytopenia one month after detection of metastasis.
CGD-TCL is a rare sub-type of peripheral cutaneous T-cell lymphoma. It is a highly aggressive tumor and is resistant to chemotherapy and radiation therapy1. In 2005, CGD-TCL was classified as a provisional entity in the WHO-EORTC classification for primary cutaneous peripheral T-cell lymphoma2. Since 2008, CGD-TCL has been included in the WHO classification as a rare sub-type of primary cutaneous peripheral T-cell lymphoma3. Lesions of this sub-type resemble those of panniculitis; therefore, it was previously known as the γ/δ sub-type of subcutaneous panniculitis-like T-cell lymphoma (SPTCL)4,5. Based on cell surface receptors on neoplastic T-cells, SPTCL was further subdivided into a α/β sub-type and a γ/δ sub-type2,5. Histopathologic findings, immunohistochemical findings, and prognosis between these 2 sub-types showed considerable difference6. Histologically, the α/β sub-type is usually limited to the subcutaneous fat; however, the γ/δ sub-type can infiltrate from the subcutaneous fat to the epidermis5. On immunohistochemical staining, the former usually has a CD4-, CD8+, and CD56- phenotype, whereas the latter commonly presents with CD4-, CD8- and CD56+5. Willemze et al.2 studied 83 cases of SPTCL in order to investigate differences between the α/β and γ/δ subtypes. Results showed development of the γ/δ sub-type in older patients, with more frequent ulceration, a more progressive disease course, rare positivity of CD8, with negativity of βF1, and greater positivity of CD56 (60% vs. 0%) and TCRδ (100% vs. 0%), compared with the α/β sub-type2. In addition, the γ/δ sub-type SPTCL is more commonly associated with hemophagocytic syndrome7 (50% vs. 17%) and a worse prognosis (5-y overall survival: 82% vs. 11%). The median survival of CGD-TCL is 15 months6. Owing to these considerable differences, and its rapidly fatal course, the γ/δ sub-type of SPTCL has been reclassified as CGD-TCL8.
Clinical features of CGD-TCL are variable: Lesions present as patches, plaques, nodules, or necrotic tumors, which are often ulcerated and primarily affect the extremities9. Patients are adults, with an equal distribution of males and females, and absence of a history of mycosis fungoides10. Presentation of these clinical features should arouse suspicion for the diagnosis of CGD-TCL. Histopathology shows proliferation of small/medium to large sized pleomorphic lymphocytes, with both marked epidermotropism and involvement of subcutaneous tissues10. Intraepidermal vesiculation, necrosis, angiocentricity, and angiodestruction can also be present10. Subcutaneous nodules may be panniculitis-like, and may show a rimming of fat cells, similar to the SPTCL α/β sub-type6. On immunohistochemical staining, tumor cells have a CD2+, CD3+, CD4-, CD5-, CD7+/-, CD8+/-, CD56+/- and β F1-phenotype, with strong expression of cytotoxic proteins, like TIA-1, granzyme B, and perforin. They also show high proliferative activity on Ki-67. TCRγ gene monoclonal rearrangement can be seen6,10. Toro et al.9 investigated factors affecting survival with cutaneous T-cell lymphoma, and showed that the γ/δ sub-type with subcutaneous involvement is predictive of a statistically significant decrease in survival9. Takeshita et al.11 reported that 5-year survival rates for subcutaneous panniculitis-like CD56+ and CD56- lymphoma were 17% and 92%, respectively. Although the study was performed before the WHO-EORTC classification was made, it informed that CD56+ is a bad prognostic factor. However, in our case, the prognosis was bad in spite of CD56 negativity. This discrepancy may be due to factors other than CD56, which may be more influential in determining the prognosis of CGD-TCL.
In addition to SPTCL, there are several other cutaneous lymphomas that need to be differentiated from CGD-TCL: Cutaneous anaplastic large cell lymphoma shows similar clinical and histological findings to CGD-TCL; however, it is composed of palisading large anaplastic cells, which show strong positivity for CD30. Furthermore, it has a favorable prognosis8. Extranodal NK/T cell lymphoma nasal type is distinguished by positivity of CD56 and EBV, and is encoded as RNA (EBER)9. Cutaneous small-medium pleomorphic T-cell lymphoma consistently shows βF1 positive results and has smaller pleomorphic cells than CGD-TCL10. Another differential diagnosis with CGD-TCL is primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, which shows βF1 positive tumor cells, and lesser involvement of the subcutaneous fat, with a lower presence of interface dermatitis10.
Patients with CGD-TCL may follow a protracted course, with resistance to currently used chemotherapeutic regimens, such as CHOP (cyclophophamid, doxorubicin, vincristine, and prednisolone) and to radiation therapy, and have a poor prognosis5,10. However, a few cases of treatment of CGD-TCL by allogenic stem cell transplantation have been reported, while treatment of CGD-TCL with retinoid and narrow band UVB12 is challenging, and will require further study.
Histological findings showed neoplastic lymphocyte infiltration into subcutaneous fat, positivity of CD3 and Granzyme B, proliferative activity of Ki-67, and negativity of the CD20 immunoperoxidase stain, although the epidermotropism was not distinct; therefore, our case was initially diagnosed as cutaneous T-cell lymphoma. After additional CD30, CD56, CD4, CD8, and βF1 staining, we made a diagnosis of CGD-TCL. Despite treatment with radiotherapy, recurrence and metastasis to the axillary node, spinal cord, and brain were observed within 4 months. Having failed to respond to treatment, the disease showed rapid progress to death by infection and multiple organ failure just 6 months after initial diagnosis. We have documented herein a rare case of fatal CGD-TCL with CNS metastasis as a reminder of the importance of suspicion, early diagnosis, and sufficiently aggressive treatment, in order to improve the prognosis.
Figures and Tables
Fig. 1
Localized, dusky red colored, centrally umbilicated plaque, with a peripheral erythematous patch, on the left foot.
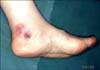
Fig. 2
(A, B) Epidermal atrophy, dermoepidermal vacuolization, massive cellular infiltration of the entire dermis, as well as a mostly lobular, inflammatory infiltrate of the subcutaneous fat of lymphoid cells and histiocytes (H&E, ×100). (C, D) At high power magnification, the "rimming" of fat cells by pleomorphic, atypical T lymphocytes in the subcutaneous fat was visible (H&E, ×400).
References
1. Koch R, Jaffe ES, Mensing C, Zeis M, Schmitz N, Sander CA. Cutaneous gamma/delta T-cell lymphoma. J Dtsch Dermatol Ges. 2009. 7:1065–1067.

2. Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, et al. EORTC Cutaneous Lymphoma Group. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008. 111:838–845.

3. Kempf W, Sander CA. Classification of cutaneous lymphomas-an update. Histopathology. 2010. 56:57–70.
4. Amado A, McDonnell JK, Somani N, Bunting ST, Winfield HL. Cutaneous gamma-delta T-cell lymphoma. Leuk Lymphoma. 2008. 49:2003–2005.

5. Kao GF, Resh B, McMahon C, Gojo I, Sun CC, Phillips D, et al. Fatal subcutaneous panniculitis-like T-cell lymphoma gamma/delta subtype (cutaneous gamma/delta T-cell lymphoma):report of a case and review of the literature. Am J Dermatopathol. 2008. 30:593–599.

6. Ralfkiaer E, Willemze R, Meijer CJLM, Dummer R, Jaffe ES, Swerdlow SH, et al. Leboit PE, Burg G, Weedon D, Sarasin A, editors. Primary cutaneous peripheral T-cell lymphoma, unspecified. Pathology and genetics of skin tumours. 2006. Lyon: IARC Press;184–188.
8. Kong YY, Dai B, Kong JC, Zhou XY, Lu HF, Shen L, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinicopathologic, immunophenotypic, and molecular study of 22 Asian cases according to WHO-EORTC classification. Am J Surg Pathol. 2008. 32:1495–1502.
9. Toro JR, Liewehr DJ, Pabby N, Sorbara L, Raffeld M, Steinberg SM, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003. 101:3407–3412.

10. Cerroni L, Gatter K, Kerl H. Skin lymphoma: the illustrated guide. 2009. 3rd ed. Chichester: Wiley-Blackwell;97–113.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download