Abstract
Eosinophic fasciitis (EF) is an uncommon connective tissue disease characterized by scleroderma-like cutaneous changes, peripheral eosinophilia, hypergammaglobulinemia, and an elevated erythrocyte sedimentation rate (ESR). Typical histopathologic findings include chronic inflammatory infiltration affecting the deep fascia with lymphocytes, histiocytes, and occasionally eosinophils. We report two cases of EF, the first of which is a 36-year-old man with a tender brownish induration on both forearms, for 2 months. Histopathologic examination showed fibrotic fascia with a mixed inflammatory cell infiltration. The second case is a 52-year-old woman with a symmetrical painful swelling and skin induration on both forearms, for 4 months. A deep biopsy demonstrated chronic inflammatory cell infiltration and hyaline degeneration in the fascia. Increased signal intensity in the fascia and tendon sheath was shown on magnetic resonance imaging. In laboratory examination, mild eosinophilia was found in both cases. Both patients had a history of physical activity (weight training and excessive housework, respectively) and showed marked improvement with high doses of oral prednisolone for several months.
Eosinophic fasciitis (EF) is an uncommon connective tissue disease, first described in 1974 by Shulman1. It is characterized by scleroderma-like cutaneous changes, peripheral eosinophilia, hypergammaglobulinemia and elevated erythrocyte sedimentation rate (ESR). The absence of Raynaud's phenomenon or sclerodactyly distinguishes EF from scleroderma. Typical histologic findings include chronic inflammatory infiltration affecting deep fascia with lymphocytes, histiocytes, and occasionally eosinophils1. The etiology and pathogenesis of EF remain to be elucidated. This report describes two cases of EF developed after excessive physical activity. They were confirmed by both deep wedge biopsy of the skeletal muscle, including the fascia, and magnetic resonance imaging (MRI), and were managed successfully with high dose corticosteroids.
A 36-year-old man presented with a 2-month history of brownish tender induration on both forearms (Fig. 1A). He had experienced immobility of both wrists. There was no history of sclerodactyly, Raynaud's phenomenon, dysphagia, dyspnea nor L-tryptophan ingestion. Chest X-ray was unremarkable. Laboratory investigations revealed white blood cell count at 6,800/mm3, with 8.1% eosinophils. ESR, liver, renal and thyroid function tests were normal. Uric acid level was 7.7 mg/dl (normal range, 2.4~7.0 mg/dl). Serology was traced for anti-nuclear antibody with mixed pattern of speckled and anticytoplasmic antibody, and was negative for anti-ds DNA antibody, anti-Scl-70 antibody, anti-centromere antibody and rheumatoid factor.
Deep tissue biopsy demonstrated fibrotic fascia with mixed infiltrate composed of lymphocytes, histiocytes, and plasma cells (Fig. 1B~D).
EF was diagnosed based on typical skin sclerosis, histopathology results and eosinophilia. He was treated with 60 mg prednisolone daily. Within 1 week, the induration softened and he experienced mildly improved joint mobility. Prednisolone dosage was reduced progressively until complete remission was achieved 2 months following diagnoses. The patient currently remains asymptomatic.
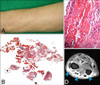
A 52-year-old female presented with symmetrical painful swelling and skin induration on both forearms for 4 months (Fig. 2A). Physical examination revealed marked tenderness of both forearms without evidence of muscle atrophy and weakness. There was no history of sclerodactyly, Raynaud's phenomenon, dysphagia, dyspnea nor L-tryptophan ingestion. Chest X-ray was normal. Hematologic evaluation demonstrated white blood cell count at 6,700/mm3 (eosinophil 7.7%), ESR at 22 mm/hr (normal range, 0~15 mm/hr), IgG 1,841 mg/dl (normal range, 700~1,600 mg/dl). Anti-nuclear antibody test was positive (titer 1:100, weak unspecified), anti-histone antibody was positive (93 U/ml, [normal range, 0~39.9 U/ml]) and anti-ds DNA antibody was negative.
Histologic examination of a deep wedge biopsy taken from the left forearm revealed thickened fascia with infiltration of lymphohistiocytes, plasma cells and hyaline degeneration (Fig. 2B, C). MRI scan revealed increased signal intensity in the fascia and tendon sheath (Fig. 2D).
Treatment with oral prednisolone was started at 60 mg daily initially. Two months later, the eosinophil count was returned to normal. The edematous changes and skin induration of both forearms gradually improved. The dosage of prednisolone was tapered over 5 months and the patient remained asymptomatic.
EF is characterized by initially edematous and erythematous extremities, followed by 'peau d'orange' and woody skin induration1. Patients have a variety of non-specific features including malaise, weakness, fever and weight loss. Laboratory investigations may reveal peripheral eosinophilia, hypergammaglobulinemia (usually IgG) and elevated ESR. Anti-nuclear antibodies, rheumatoid factor and anti-ds DNA antibodies may be present. It occurs equally in males and females, and most patients are in their third to sixth decades2. Pediatric disease, however, has also been documented3. EF has been frequently associated with hematological disorders such as aplastic anemia, hemolytic anemia, peripheral autoimmune thrombocytopenia, pernicious anemia, leukemias, and lymphomas4,5. Some autoimmune diseases such as Hashimoto's thyroiditis, morphea, systemic sclerosis, Sjogren's syndrome and antiphospholipid antibody syndrome have also been reported in patients with EF6.
The etiology and pathogenesis are unknown. At least 66% of patients relate the onset of the disease to previous excessive physical activity7,8. In the two cases discussed above, there had been a history of vigorous physical activity (weight training [case 1], and excessive housework [case 2]). Blaser et al.8 suggested that excessive exercise or trauma may trigger antigenicity of the fascia and subcutis. Other hypothesized triggers include trauma9, phenytoin10, trichloroethylene11 and Borrelia burgdorferi infection12. EF is confirmed by histopathologic findings. Typical findings are: thickening of the fascia associated with inflammatory cell infiltration consisting of lymphocytes, plasma cells, histiocytes, and eosinophils in variable degrees. The presence of eosinophils in the fascia is not confirmatory of the diagnosis7,13. Additionally, Baumann et al.14 suggested that MRI in EF demonstrates typical findings, including abnormal signal intensity, thickening and contrast enhancement of the fascia. Therefore, MRI can facilitate diagnostic investigations, guide biopsy site and evaluate therapeutic response.
The differential diagnosis of EF includes morphea, systemic sclerosis and eosinophilia-myalgia syndrome. In morphea, the inflammatory process predominantly affects the reticular dermis and superficial subcutis, but EF is characterized by involvement of the deep subcutis and fascia7. The epidermis, papillary dermis and adnexa are not affected13. In contrast to systemic sclerosis, sclerodactyly and Raynaud's phenomenon, internal organ involvement are absent in EF. Eosinophilia-myalgia syndrome has similar findings, both clinically and histologically, with EF, but has a history of L-tryptophan ingestion, polyneuropathy and pulmonary symptoms13. Our patients have classical EF histories and investigation outcomes: the onset was rapid after vigorous physical activity, with peripheral eosinophilia and hypergammaglobulinemia. They did not experience sclerodactyly, Raynaud's phenomenon, dysphagia, dyspnea and L-tryptophan ingestion. Their epidermis, papillary dermis and adnexa were not affected. The fascia of both patients showed thickening or fibrosis with inflammatory cell infiltration.
The gold standard of EF treatment is high-dose corticosteroids, reported to be effective in up to 70% of cases7. Other therapeutic regimens include hydroxychloroquine, ketotifen, cimetidine, methotrexate, cyclosporine, penicillamine, azathioprine and griseofulvin15, but patient response has been limited. Recently, Weber et al.16 reported that a combination regimen, consisting of UVA1 phototherapy, isotretinoin and corticosteroid, led to good clinical outcomes. The supposed effects of isotretinoin are inhibition of fibroblast growth and reduction of collagen production in dermal fibroblasts. The likely effects of UVA1 phototherapy on matrix metalloproteinase-1, and that of prednisone as an anti-inflammatory effect make this combination regimen an attractive option for managing EF, requiring lower steroid dosage and treatment time.
The clinical outcome of EF is usually good, except when it is associated with a malignant hematologic disease. Endo et al.17 suggested that young age (under 12 years) at onset, trunk involvement and morphea-like skin sclerosis might be a hallmark of refractory disease. Jinnin et al.18 reported that EF patients have significantly higher serum tissue inhibitor of metalloproteinase-1 (TIMP-1) level than does a healthy person. Therefore, TIMP-1 may be implicated in the pathogenesis of EF, which would be a good serological marker for disease activity, similar to gammaglobulins.
In summary, a deep wedge biopsy of the skeletal muscle, including the fascia, should be performed for diagnosis. In addition, MRI scan may be helpful in diagnosing atypical EF cases, when biopsy is unavailable or when the result of biopsy is doubtful; it can also readily monitor disease activity and therapeutic effect. Initial high dose steroid treatment is crucial in decreasing disease activity and duration.
Figures and Tables
Fig. 1
(A) Tender, brownish induration on both forearms. (B) A deep tissue biopsy specimen from the lesion shows chronic inflammation with fibrosis (H&E, ×25). (C) The fascia is fibrotic with mixed inflammatory cell infiltration (H&E, ×100). (D) Mixed inflammatory cell infiltration composed of lymphohistiocytes and plasma cells (H&E, ×400).
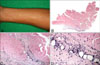
Fig. 2
(A) Symmetrical, painful swelling and skin induration on both forearms. (B) The fascia shows thickening with chronic inflammatory cell infiltration and hyaline degeneration (H&E, ×25). (C) Infiltration of lymphohistiocytes and plasma cells in the fascia (H&E, ×400). (D) Contrast-enhanced fat-suppressed T1-weighted axial magnetic resonance image of the left wrist shows strong enhancement along the superficial and deep intermuscular fascia, extensor and flexor tendon sheath (arrows).
References
1. Shulman LE. Diffuse fasciitis with hypergammaglobulinemia and eosinophilia: a new syndrome? [abstract]. J Rheumatol. 1974. 1:Suppl. 1. 46.
3. Grisanti MW, Moore TL, Osborn TG, Haber PL. Eosinophilic fasciitis in children. Semin Arthritis Rheum. 1989. 19:151–157.

4. Narayanan MN, Liu Yin JA, Love EM, Geary CG, Holt PJ, Freemont AJ, et al. Eosinophilic fasciitis and aplastic anaemia. Clin Lab Haematol. 1988. 10:471–474.

5. Chan LS, Hanson CA, Cooper KD. Concurrent eosinophilic fasciitis and cutaneous T-cell lymphoma. Eosinophilic fasciitis as a paraneoplastic syndrome of T-cell malignant neoplasms? Arch Dermatol. 1991. 127:862–865.

6. Castanet J, Lacour JP, Perrin C, Taillan B, Dubois D, Ortonne JP. Association of eosinophilic fasciitis, multiple morphea and antiphospholipid antibody. Dermatology. 1994. 189:304–307.

7. Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988. 17:221–231.

8. Blaser KU, Steiger U, Würsch A, Speck B. Eosinophilic fasciitis with aplastic anemia and Hashimoto's thyroiditis. Review of the literature and report of a typical example. Schweiz Med Wochenschr. 1989. 119:1899–1906.
9. Romero AG, Fernandez JG, Calatayud JC. Eosinophilic fasciitis associated with simple traumatism. Acta Dermatovenerol Croat. 2001. 9:287–290.
10. Buchanan RR, Gordon DA, Muckle TJ, McKenna F, Kraag G. The eosinophilic fasciitis syndrome after phenytoin (dilantin) therapy. J Rheumatol. 1980. 7:733–736.
11. Hayashi N, Igarashi A, Matsuyama T, Harada S. Eosinophilic fasciitis following exposure to trichloroethylene: successful treatment with cyclosporin. Br J Dermatol. 2000. 142:830–832.

12. Granter SR, Barnhill RL, Duray PH. Borrelial fasciitis: diffuse fasciitis and peripheral eosinophilia associated with Borrelia infection. Am J Dermatopathol. 1996. 18:465–473.

13. Antic M, Lautenschlager S, Itin PH. Eosinophilic fasciitis 30 years after - what do we really know? Report of 11 patients and review of the literature. Dermatology. 2006. 213:93–101.

14. Baumann F, Brühlmann P, Andreisek G, Michel BA, Marincek B, Weishaupt D. MRI for diagnosis and monitoring of patients with eosinophilic fasciitis. AJR Am J Roentgenol. 2005. 184:169–174.

15. Fuchs BS, Gordon ML. Lebwohl MG, Heymann WR, Berth-Jones J, Conlson I, editors. Eosinophilic fasciitis. Treatment of skin disease: comprehensive therapeutic strategies. 2006. 2nd ed. London: Mosby;180–182.
16. Weber HO, Schaller M, Metzler G, Röcken M, Berneburg M. Eosinophilic fasciitis and combined UVA1-retinoid-corticosteroid treatment: two case reports. Acta Derm Venereol. 2008. 88:304–306.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download