Abstract
Pigmented mammary Paget disease (PMPD) is a rare subtype of mammary Paget disease that presents as a hyperpigmented patch or plaque over the areola and nipple. We herein report on an unusual case of PMPD with reticulated features in a 98-year-old female. The histology showed intraepidermal pagetoid cells containing melanin pigments without any underlying intraductal carcinoma.
Pigmented mammary Paget disease (PMPD) is a rare variant of mammary Paget disease (MPD), and MPD and PMPD resembles eczema or melanoma. It typically involves the nipple, but it is rarely confined to the areola complex. The clinically visible pigmentation in PMPD is due to the abundant melanin granules dispersed in the epidermis and dermis. A thorough histopathologic examination is mandatory to rule out malignant melanoma as it is clinically and histopathologically similar to PMPD. We herein describe a unique case of PMPD with a reticulated pattern. To the best of our knowledge, this is the first report of PMPD with a reticulated pattern.
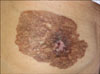
A 98-year-old Korean female presented with a dark brown colored reticulated plaque on the left breast and this lesion was first detected a year previously (Fig. 1). The lesion was about 10×8 cm in size, and the normal anatomical structure of the nipple was destroyed. There was no palpable mass or axillary lymphadenopathy. The patient had no previous history of breast cancer.
The specimen taken from the areolar complex showed the histological findings of large clear cells with vesicular nuclei and these cells were dispersed in the suprabasal layers of the epidermis, without evidence of dermal invasion (Fig. 2). The cells were intermingled with numerous melanin-rich melanocytes. Other than a mild lymphohistiocytic infiltration in the upper dermis and an increased number of melanophages, the dermis was normal with no apparent malignant cells. Immunohistochemical staining was done to identify the nature of the large clear cells. The cells were strongly positive for anticytokeratin 7, c-erbB-2 and CEA, whereas they were negative for protein S-100 and HMB-45. The findings were consistent with Paget cells, and so the diagnosis of MPD was made. However, unlike the Paget cells in the previous reports, some cells in our case contained dusty melanin pigments and these cells stained focally positive for S-100 protein and HMB-45, and there was an increased number of melanocytes in the epidermis and melanophages in the dermal stroma. Based on the findings, a final diagnosis of PMPD was made. Excision and further evaluation was recommended, but the patient refused further treatment and was lost to follow-up.
Paget cells with abundant melanin have been previously reported on. However, it was only in 1990 that the term PMPD was used to describe the rare pigmented variant of MPD1. Only a few other cases of PMPD have been reported since then. Compared to the typical MPD that usually presents as an erythematous eczematoid patch, PMPD is unique in that the lesion is clinically hyperpigmented. Unfortunately, this feature makes differentiation between PMPD and malignant melanoma difficult and even on a dermoscopic and reflectance-mode confocal microscopic examination2. Therefore, performing biopsy and immunohistochemical studies are mandatory for making the proper diagnosis.
The main clinicopathologic differential diagnosis of PMPD is melanoma. Apart from the fact that melanoma rarely involves the nipple, there are several histologic features that differentiate PMPD from melanoma3,4. In the latter, large vacuolated neoplastic cells are scattered through the epidermis and they usually border directly on the dermis, with occasional invasion to the dermis. On the other hand, the Paget cells of PMPD usually have a suprabasal distribution without dermal invasion. Due to the occasional presence of melanin-containing Paget cells, immunohistochemical staining is mandatory for making the proper diagnosis. Unlike the tumor cells in melanoma that contain abundant cytoplasmic protein S-100 and which stain strongly positive for HMB-45, the melanin-containing Paget cells are only focally positive for cytoplasmic protein S-100 and HMB-45. Another distinguishing factor is the negative staining for anticytokeratin 7 in melanoma cells5. On the other hand, in a recent report, anticytokeratin 7 was positive in virtually 100% of the MPD cases3. Anticytokeratin 7 is also known to be a sensitive, but not specific marker for Paget disease because it is also positive in other tumors such as colorectal and salivary gland tumors6.
The etiology of hyperpigmentation in PMPD is largely unknown, yet several theories have been proposed. One theory suggests that the local production of melanocytic chemoattractant factors by neoplastic Paget cells induces melanocytic proliferation7. Another theory suggested, based on several reports showing dusty melanin granules in Paget cells even without an increased number of melanocytes, the possibility of phagocytosis or transfer of melanin from melanocytes to the intraepidermal neoplastic cells.
Other localized reticulate pigmentary disorders occurring on the breasts include erythema ab igne prurigo pigmentosa, post-inflammatory hyperpigmentation secondary to contact dermatitis and hyperpigmentation due to some chemotherapeutic agents such as bleomycin or 5-fluorouracil8. Like the other pigmentary abnormalities, the pathogenesis of the unusual reticulated pattern seen in this case remains unclear. However, some theories suggest pigmentation through an action of melanocyte-stimulating hormone (MSH) and adrenocorticotrophic hormone. The MSH accelerates the production of melanin by increasing cyclic AMP from normal melanocytes. Further, the high estrogen level associated with an underlying breast cancer could cause hyperpigmentation on small areas of the body, as happens during pregnancy or when taking oral contraceptives9.
In summary, we have presented a case of PMPD in a female patient. In contrast to all the other previous cases that presented with hyperpigmented macules, patches or plaques, our case is unique in that the hyperpigmentation was in a fishnet-like pattern.
Figures and Tables
References
1. Ho TC, St Jacques M, Schopflocher P. Pigmented Paget's disease of the male breast. J Am Acad Dermatol. 1990. 23:338–341.

2. Longo C, Fantini F, Cesinaro AM, Bassoli S, Seidenari S, Pellacani G. Pigmented mammary Paget disease: dermoscopic, in vivo reflectance-mode confocal microscopic, and immunohistochemical study of a case. Arch Dermatol. 2007. 143:752–754.
3. Petersson F, Ivan D, Kazakov DV, Michal M, Prieto VG. Pigmented Paget disease--a diagnostic pitfall mimicking melanoma. Am J Dermatopathol. 2009. 31:223–226.
4. Requena L, Sangueza M, Sangueza OP, Kutzner H. Pigmented mammary Paget disease and pigmented epidermotropic metastases from breast carcinoma. Am J Dermatopathol. 2002. 24:189–198.

5. Fujisawa Y, Yamamoto A, Machida H, Yamazaki N. Cytokeratin 7 staining was useful in a case of pigmented mammary Paget's disease resembling malignant melanoma. Int J Dermatol. 2006. 45:1257–1258.

6. Kirkham N. Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Tumors and cysts of the epidermis. Lever's histopathology of the skin. 2009. 10th ed. Philadelphia: Lippincott Williams & Wilkins;838–839.
7. Konomi K, Imayama S, Nagae S, Terasaka R, Chijiiwa K, Yashima Y. Melanocyte chemotactic factor produced by skin metastases of a breast carcinoma. J Surg Oncol. 1992. 50:62–66.

8. Allen BJ, Parker D, Wright AL. Reticulate pigmentation due to 5-fluorouracil. Int J Dermatol. 1995. 34:219–220.

9. Jimbow K, Fitzpatrick TB, Wick MM. Goldsmith LA, editor. Biochemistry and physiology of melanin pigmentation. Physiology, biochemistry, and molecular biology of the skin. 1991. 2nd ed. New York: Oxford University Press;873–909.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download