Abstract
Disseminated superficial actinic porokeratosis (DSAP) consists of multiple annular, hyperkeratotic lesions that have a bilateral distribution on sun-exposed areas, particularly the extremities. DSAPs have a wider distribution than porokeratosis of Mibelli and usually develop during the 3rd or 4th decade of life. Squamous cell carcinoma that arises in the classical type of porokeratosis of Mibelli is well-documented, but there are only a few reports of squamous cell carcinoma in DSAP. Here, we describe a 62-year-old man with DSAP who developed squamous cell carcinoma on his right forearm.
Disseminated superficial actinic porokeratosis (DSAP) is the most common form of porokeratosis, and is inherited as an autosomal dominant condition, with reduced penetrance at a younger age1. The characteristic lesions of DSAP represent multiple annular, hyperkeratotic, brownish macules measuring 2~5 mm in diameter. The center of a macule is minimally atrophic or depressed and the border spreads centrifugally in raised ridges1,2. The distribution is symmetric and usually affects sun-exposed areas.
A 62-year-old male presented with pruritic eruptions on sun-exposed portions of both forearms that had gradually increased in number over a period of 5 years (Fig. 1). The lesions were exacerbated during the summer months. Along with these lesions, an erythematous, irregular, marginated, scaly, crusted plaque had developed on the right forearm 3 years earlier. There was no significant medical or family history.
On physical examination, a 2×3 cm erythematous, irregular, marginated, scaly, crusted plaque was noted on the right forearm. In addition, the patient had numerous annular, brown, atrophic, and symmetric macules surrounded by well-demarcated, raised ridges on extensor aspects of both forearms, which are characteristics of DSAP. Complete blood count, as well as liver and kidney function tests were all within normal limits.
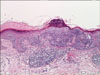
A skin biopsy specimen of the multiple, brown, annular lesions showed histologic changes of typical DSAP. There was a cornoid lamella composed of a column of parakeratosis with underlying hypogranulosis and perivascular lymphocytic infiltrations in the dermis localized beneath the cornoid lamella. A skin biopsy obtained from the erythematous plaque on the right forearm showed dysregulated keratinocytes with hyperchromatic, atypical nuclei, consistent with squamous cell carcinoma. A cornoid lamella was observed in the lesion of the squamous cell carcinoma (Fig. 2).
Positron emission tomography-computed tomography revealed no evidence of distant metastasis. The squamous cell carcinoma was treated by total excision and split-thickness skin graft and radiotherapy. The patient is currently being treated with topical sunscreens.
Porokeratosis has several clinical varieties including porokeratosis of Mibelli, giant porokeratosis, DSAP, porokeratosis palmaris et plantaris, punctuate porokeratosis, and linear porokeratosis8. The most common form of porokeratosis is DSAP, which was first described as a clinical entity by Chernosky in 1966. The distribution of typical lesions is symmetric and usually affects sun-exposed areas. The lesions generally spare the face, palms, soles, and mucosal surfaces1,7. DSAP can affect people of all ages, but manifests during the 3rd or 4th decade of life.
The pathogenesis of DSAP is not clearly understood, but frequent p53 overexpression in the epidermis of porokeratotic lesions have been detected9. Overexpression of p53 can be induced by p53 gene mutations and other DNA damaging agents, such as ultraviolet (UV) light and ionizing radiation. In a previous study, mutations of the p53 gene were not detected in porokeratotic lesions9. This finding suggests that other causative mechanisms exist for overexpression of p53 in the epidermis of porokeratotic lesions. The patient in this case showed overexpression of p53 in the epidermis of DSAP lesions (Fig. 3).
The occurrence of malignancies in porokeratotic lesions is clinical evidence of the pre-cancerous nature of this disease. Malignancies have been reported for porokeratosis of Mibelli, linear porokeratosis, porokeratosis palmaris et plantaris and DSAP3-7,10,11. Associated malignancies are squamous cell carcinoma, Bowen's disease and basal cell carcinoma. Squamous cell carcinoma arising in the classic type of porokeratosis of Mibelli is well-documented, but there are only a few reports of squamous cell carcinoma in DSAP3-7. All of the reported squamous cell carcinoma cases arising from DSAP lesions have originated in the distal extremities. These findings indicate that there is a significant role of UV light on the evolution of squamous cell carcinoma from DSAP. In addition, results from previous reports show that p53 gene mutations are responsible for the progression of porokeratosis to SCC in at least some cases9.
In our case, a cornoild lamella was observed in the squamous cell carcinoma, strongly suggesting the possibility that the carcinoma developed from clones of abnormal epithelial cells. However, not all cases of squamous cell carcinoma in DSAP have cornoid lamellae in lesions of the carcinoma6. These cases cannot exclude the possibility that squamous cell carcinoma developed simply from chronic sun exposure unrelated to DSAP lesions. However, even when a cornoid lamella directly over the tumor is not identified, the presence of a cornoid lamella in close proximity to the tumor suggests the possibility that the carcinoma has arisen from the DSAP lesions6,7.
Porokeratosis does not usually need treatment, but in some cases, treatment is necessary due to potential for progression to a malignancy and for cosmetic purposes. In contrast, treatment of DSAP lesions is unsatisfactory. As in this case, the potential for progression to cancer induced by UV light exists, thus sun avoidance must be emphasized when treating DSAP.
The prognosis of squamous cell carcinoma in DSAP lesions has not been reported yet. However, the reported cases have had no recurrences after excision of the cancerous lesions.
Figures and Tables
Fig. 1
An irregular, marginated, erythematous plaque and multiple, brown, atrophic macules surrounded by well-demarcated, raised ridges on the right forearm.
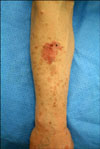
Fig. 2
Biopsy specimen obtained from the erythematous plaque on the right arm. In epidermis, acanthosis and dysregulated keratinocytes with hyperchromatic, atypical nuclei are observed. A cornoid lamella composed of a column of parakeratosis is seen in the lesion of the squamous cell carcinoma (H&E, ×100).
References
1. Shumack SP, Commens CA. Disseminated superficial actinic porokeratosis: a clinical study. J Am Acad Dermatol. 1989. 20:1015–1022.

2. Schwarz T, Seiser A, Gschnait F. Disseminated superficial "actinic" porokeratosis. J Am Acad Dermatol. 1984. 11:724–730.

3. Yang HY, Nam TS, Kim YT, Kim JH. A case of squamous cell carcinoma and Bowen's disease associated with superficial disseminated porokeratosis. Ann Dermatol. 1990. 2:31–34.

4. James WD, Rodman OG. Squamous cell carcinoma arising in porokeratosis of mibelli. Int J Dermatol. 1986. 25:389–391.

5. Shrum JR, Cooper PH, Greer KE, Landes HB. Squamous cell carcinoma in disseminated superficial actinic porokeratosis. J Am Acad Dermatol. 1982. 6:58–62.

6. Chernosky ME, Rapini RP. Squamous cell carcinoma in lesions of disseminated superficial actinic porokeratosis: a report of two cases. Arch Dermatol. 1986. 122:853–855.

7. Leache A, Soto de Delás J, Vázquez Doval J, Lozano MD, Quintanilla E. Squamous cell carcinoma arising from a lesion of disseminated superficial actinic porokeratosis. Clin Exp Dermatol. 1991. 16:460–462.

8. Won JH, Lee MJ, Park JS, Chung H. A case of the giant and hyperkeratotic variant of porokeratosis. Korean J Dermatol. 2009. 47:101–103.
9. Ninomiya Y, Urano Y, Yoshimoto K, Iwahana H, Sasaki S, Arase S, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997. 14:173–178.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download