INTRODUCTION
CD20 positive T cell lymphoma is a rare condition that is characterized by the coexpression of CD20 and T cell markers, such as, CD3, CD5, or UCHL-11. Positivity for CD20 in any type of T cell lymphoma represents an aberrant immunophenotype, despite the presence of various indicators of T cell lymphoma.
CD20 is a transmembrane protein, with a molecular weight of 35 to 37 kd, which is expressed early during B cell development and lost during terminal B cell differentiation into plasma cells1. Its function is still unclear but is suspected to act as a calcium channel in the cell membrane. CD20 is classified as a pan-B cell marker, and its presence on benign and neoplastic lymphocytes is generally considered specific for B-lineage. However, recent studies have indicated that peripheral T cell lymphomas rarely express CD202-10, and because CD20 expression in T cell lymphoma is rare, obtaining a correct diagnosis of this type of CD20 positive lymphoma can be difficult. Accordingly, because misdiagnosis has a substantial impact on therapeutic strategy, careful morphologic evaluation and wide range of immunophenotypic tools and molecular genetic studies must be employed to achieve an accurate diagnosis.
Here, we report the case of a 56-year-old man with CD20 positive peripheral T cell lymphoma who had been mistakenly diagnosed as having B cell type lymphoma 4 years previously.
CASE REPORT
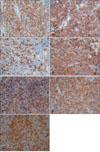
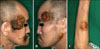
A 56-year-old Korean man visited our department in March 2009 with a 1-year history of multiple crusted erythematous masses on his face, neck, and left forearm (Fig. 1). The patient did not manifest any symptoms, such as, itching, pain, tenderness, fever, weight loss, or night sweats. In a previous visit to a community hospital in 2005, he had been diagnosed as having B cell type lymphoma based on the results of a surgical biopsy performed on an enlarged lymph node in his neck. Subsequently, he underwent 9 cycles of CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone), which resulted in clinical remission. However, in 2008, small-sized reddish papules appeared on his face and grew in size with a firmer consistency than the original erythematous masses. New lesions also subsequently developed at other skin sites. His clinical differential diagnosis was indeterminate but skin metastasis or squamous cell carcinoma was suspected. A skin biopsy was then performed on a neck mass.
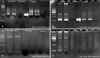
Histopathologically, basophilic tumor cells were found to infiltrate diffusely into the deep dermis without epidermal involvement. The small to medium sized round tumor cells contained vesicular nuclei, prominent nucleoli, and adopted a relatively monotonous appearance (Fig. 2). Immunohistochemically, the neoplastic cells were positive for CD3, CD4, CD5, UCHL-1, CD20, CD79a, and bcl-2 (Fig. 3). However, CD10, CD30, CD56, CD68, CD138, granzyme B, and EBV in situ were not expressed, although normal lymphocytes were positivite for CD8 and TIA-1.
Complete blood cell count, differential cell count, urinalysis, and liver function testing (including BUN/Cr) were normal as well as peripheral blood smear and a bone marrow biopsy were also normal. Computerized tomography revealed multiple enlarged tumor masses on the neck with lymphadenopathies on the interjugular, submandibular, and submental lymph nodes.
We determine the nature of this case, where both T and B cell associated antigens were expressed, by performing multiplex PCR studies to assess the rearrangement of T cell receptor (TCR) gamma and immunoglobulin heavy chain (IgH). In addition, we histopathologically reviewed the biopsy specimen taken in 2005 from a neck lymph node, and subjected it to immunophenotypic and genotypic analysis.
For the genotypic analysis, 35 cycles of two multiplex PCR reactions were performed for TCR gamma gene rearrangements on the DNA extracted from 8 µm sections that were deparaffinized. The primers used in Mix 1 PCR were: V2(5'-CTT-CCT-GCA-GAT-GAC-TCC-TAC-AAC-TCC-AAG-GTT G-3'), V3(5'-CTT-CCT-GCA-GAT-GAC-GTC-TCC-ACC-GCA-AGG-GAT-G-3'), V4(5'-CTT-CCT-GCA-GAT-GAC-TCC-TAC-ACC-TCC-AGC-GTT-G-3'), V8(5'-CTT-CCT-GCA-GAT-GAC-TCC-TAC-AAC-TCC-AGG-GTT-G-3'), V9(5'-GGN-ACT-GCA-GGA-AAG-GAA-TCT-GGC-ATT-CCG-3'), JGT12(5'-AAG-TGT-TGT-TCC-ACT-GCC-AAA-3'), JGT3 (5'-AGT-TAC-TAT-GAC-CTA-GTC-CC-3'), JGT4(5'-TGT-AAT-GAT-AAG-CTT-TGT-TCC-3'). The primers used in the Mix 2 PCR were: V5(5'-TTC-CTG-CAG-ATG-ACG-TCT-CCA-ACT-CAA-AGG-ATG-3'), V10(5'-CTC-TGC-AGA-ATC-CGC-AGC-TCG-ACG-CAG-CA-3'), V11(5'-CAC-TGC-AGG-CTC-AAG-ATT-GCT-CAG-GTG-GG-3'), V12(5'-ACT-CTG-CAG-CCT-CTT-GGG-CAC-TGC-TCT-AAA-3') and the same three joining primers. Jurkat cells were used as positive controls and a previous negative sample was used as a negative control. Thirty-five cycles of semi-nested PCR for the FRIII-J segment were conducted for IgH gene rearrangements, using the primers FRIIIA(5'-ACA-CGG-CYS-TGT-ATT-ACT-GT-3'), LJH(5'-TGA-GGA-GAC-GGT-GAC-C-3'), and VLJH(5'-GTG-ACC-AGG-GTN-CCT-TGG-CCC-CAG-3'). The semi-nested first primers were FRIIIA and LJH and the second primers were FRIIIA, VLJH. RAJI cells were used as positive controls and a previous negative sample was used as a negative control. The TCR gamma gene rearrangement showed monoclonality at around 200bp in our and the previous biopsy specimens, but the IgH gene rearrangement showed no monoclonality in either specimen (Fig. 4).
Histopathologic findings of the neck lymph node biopsy performed in 2005 revealed the presence of basophilic small to medium sized tumor cells infiltrating in a diffuse manner that sometimes formed multiple nodules and effacement of the normal architecture, which resembled that observed in our skin specimen. The immunophenotypic evaluation was strongly positive for CD20 and weakly reactive to UCHL-1 in the cytoplasm of atypical lymphocytes (Fig. 5). In addition, neoplastic cells were found to be positive for CD3 and CD4 and negative for CD30 (Fig. 6).
All these findings were compatible with CD20 positive peripheral T cell lymphoma. Supposedly, the disease had recurred in the skin from systemic disease or metastasized from nodal disease. The patient was treated with ifosfamide, methotrexate, VP-16 (etoposide), and prednisolone chemotherapy regimen and showed partial remission and reduction in mass size (Fig. 7).
DISCUSSION
When CD20 is expressed by T cell lymphoma, dermatologists or pathologists find it difficult to reach a correct diagnosis. Although CD20 is a pan-B cell marker, it can be expressed in some T cell malignancies, as has been reported recently2-10. The entity, described as CD20 positive T cell lymphoma, has the following characteristic features; positive for the CD20 antigen, expression of one or more pan-T-cell antigens (CD2, CD3, CD5, or CD7), and monoclonal rearrangements of TCR γ or β without rearrangement of the IgH gene2-10.
CD20 positive T cell lymphoma is extremely rare and fewer than 40 cases have been reported11-15. A review of the literature revealed that the majority of the reported cases involved the lymph node and extranodal involvement was uncommon. CD20 positive T cell lymphoma with only skin involvement accounts for 6/35 cases (17%) where the skin involvement manifests as cutaneous plaques, nodules, or erythematous patches11.
Several hypotheses have been proposed to explain the nature of CD20 positive T cell lymphoma. The first hypothesis is that normal circulating CD20 positive T cells may undergo neoplastic transformation6, the second is that CD20 positive cells may react with normal B cells distributed among neoplastic T cells, and the third is that CD20 may be a marker of normal T cell activation16,17. Based on the consistently of results regarding the presence of TCR gene rearrangement and the lack of evidence of transformation from B cell lymphoma at the two time points in the described case, we believe that initially circulating normal CD20+ T cell subsets underwent neoplastic transformation, and that CD20 positive T cell lymphoma subsequently developed in a lymph node. Furthermore, CD30, another activation marker, was not expressed at any time in the described case, despite the fact that the atypical tumor cells were positive for CD20.
We need to discern this disease from another entity of lymphomas. T cell-rich B cell lymphoma can be considered when less than 10% of large neoplastic B cells are admixed with abundant normal reactive T cells in the same specimen. However, IgH gene rearrangement studies on tissue samples usually show monoclonality18. Anaplastic large cell lymphoma can be discriminated by the presence of large cells with horseshoe shaped nuclei that are CD30 positive19. In addition, neoplastic T cells in primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma usually are CD3+CD4+ CD8-CD30- and can be admixed with CD20 positive B cells, and thus, it is difficult to differentiate this disease from CD20 positive T cell lymphoma.
Previous studies have shown that rituximab (a humanized anti-CD20 monoclonal antibody) can be used to treat CD20 positive B cell lymphoma. Rituximab can kill CD20+ cells directly due to its complement-mediated and antibody-dependent cell-mediated cytotoxicities, and indirectly by inducing structural changes, apoptosis, and by sensitizing cancer cells to chemotherapy20. Accordingly, the anti-CD20 monoclonal antibody, which selectively targets CD20, could provide a means of treating CD20 positive T cell lymphoma.
Although the role of the CD20 antigen in T cell lymphomas has yet to be determined, the majority of CD20 positive T cell lymphoma cases have been reported as peripheral T cell lymphoma not otherwise specified11,21,22. We believe that a comprehensive study on the pathogenesis of CD20 positive T cell lymphoma and follow-up of more cases will result in a re-classification of this disease as a new disease entity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download