Abstract
Classic Kaposi sarcoma (KS) is a rare human herpes virus 8-associated angioproliferative disease, and the disseminated classic type of KS in Korea is even rarer. The treatment options for classic KS vary and range from surgical excision to ionizing irradiation or chemotherapy. Recently, there have been a few reports of treating classic KS with paclitaxel, which has been used to treat AIDS-associated KS and post-transplant KS. We herein report a case of disseminated classic type KS in a 78-year-old Korean male patient who showed dramatic response after only two cycles of paclitaxel treatment.
Kaposi's sarcoma (KS) is rare type of visceral and soft tissue sarcoma related to human herpes virus (HHV)-8 that usually occurs in elderly men1. It is characterized by the proliferation of spindle shaped cells, mainly of vascular origin. Proliferation of those cells is considered to be the neoplastic elements of KS, along with neoangiogenesis, inflammatory cell infiltration and edema2. Among the four types of KS, treatment of the classic type KS varies, depending on the progression of disease and age of the patients3,4. Usually, localized classic type KS is effectively treated by radiotherapy or surgery, but disseminated classic type KS is treated by controversial methods such as radiotherapy, chemotherapy, and immunomodulatory agents5. Taxanes (paclitaxel or docetaxel) have been used for the treatment of various tumors, including ovarian, breast, and lung carcinomas6. Moreover, several studies have shown that paclitaxel monotherapy is a successful second-line treatment for both AIDS-associated KS7 and KS in a therapeutically immunosuppressed patient8. Reports about the clinical efficacy of taxanes (paclitaxel or docetaxel) are anecdotal, and treatment of disseminated classic KS with paclitaxel in Korea is very rare. Below, we report a patient with disseminated classic type KS who showed a dramatic response to paclitaxel therapy.
A 78-year-old man had developed a purpuric plaque on his left thumb 7-month ago. Progressive spreading violaceous plaques with dark purple disseminated nodules appeared on his extremities, and lymphedematous change was noted on the left arm (Fig. 1A). He had no history of trauma to the left thumb or treatment history with corticosteroids or anticoagulation drugs.
The biopsy of his arm disclosed no specific change in the epidermis, but the whole dermis was composed of extensive hemorrhaging and the presence of irregular anastomosing vascular channels lined by atypical, enlarged endothelial cells permeated by collagen bundles. Immunohistochemical staining for CD 31 and CD 34 were positive in the endothelial cells and the spindle-shaped cells in the dermis (Fig. 2). The clinical and pathologic features indicated that the diagnosis was consistent with KS.
Laboratory tests for the human immunodeficiency virus or immunosuppression were all negative. Computed tomography scans of the neck, chest and abdomen revealed no metastatic lesions, and the brain magnetic resonance imaging revealed no evidence of metastasis. At multidisciplinary review, we planned paclitaxel monotherapy, which has been proved to be effective and well tolerated in patients with aggressive refractory classic KS, because the patient was elderly. We chose paclitaxel (Genexol-PM; Samyang, Seoul, Korea) as a monotherapy to reduce complications and improve the quality of life. Paclitaxel was administered intravenously at a dose of 135 mg/m2 once every three weeks. After two infusions, the KS lesions showed dramatic improvement, with improvement of the hyperpigmentation and edema in the extremities (Fig. 1B). Though the patient required additional treatment, we could not continue the paclitaxel because of his old age and poor general condition. During the six-month follow up period, the patient developed no additional KS lesions.
The pathogenesis of KS is multifactorial, including HHV8, inflammatory cytokines, basic fibroblast growth factor, vascular endothelial growth factor, the proteins encoded by the HHV8 latency genes, matrix metalloproteinases, oncogenes, and oncosuppressor genes that induce initiation and/or progression3. There are four variant clinical and epidemiological forms of KS that have been recognized: Classic KS or Mediterranean KS, Endemic KS or African KS, KS in therapeutically immunosuppressed patients, and AIDS associated KS (AIDS-KS).
Classic KS is an uncommon disease among middle aged and elderly men of Mediterranean or Jewish lineage9,10. In Korea, Classic KS occurrences are rare, especially the disseminated classic type of KS. Classic KS usually starts to appear on the skin of the lower extremities, where it is frequently misdiagnosed as a bruise. As time progresses, the lesions increase in size and number, and the color gets darker. Early diagnosis is paramount to decrease metastasis to other organ systems such as the lungs, kidneys, and liver11.
Therapeutic options for KS are based upon disease stage, progression pattern and distribution, clinical type, and immune status12,13. For KS patients with more widely disseminated, progressive or symptomatic disease, systemic therapy with cytotoxic chemotherapy is generally warranted14. Vinblastine and bleomycin or vinblastine alone can be considered to be first-line therapy in Classic KS15. Those regimens have shown promising results in patients with severely compromised immune function (leucopenia and neutropenia are quite frequent). Other systemic medications that are Food and Drug Administration approved are liposomal doxorubicin, paclitaxel, and interferon-α16. However, since liposomal doxorubicin was not available in Korea, and interferon-α caused immediate toxicity (chills, fever and malaise), those therapies were not chosen for this study. Paclitaxel has a powerful anti-tumor effect, used in the treatment of several carcinomas arising from the ovary, breast, and lungs. Paclitaxel actively impact the microtubules and cellular vital processes in non-mitotic phases of the cell cycle and inhibits the growth of either rapidly or slowly proliferating tumors17. Paclitaxel has also been used successfully in AIDS-associated KS victims who failed to previously respond to systemic chemotherapy.
There have been some anecdotal reports on the efficacy of paclitaxel in non-HIV-associated forms of KS, and, only recently, there was a report about the tolerability and clinical efficacy of paclitaxel in a homogeneous group of advanced aggressive classic KS15,18. The protocol of the above-mentioned studies differs from this study. The results of paclitaxel in those studies were analogous to our study, however, and our patient showed rapid response after two cycles. Although we had to end the treatment after two cycles, the results are still significant considering the age of the patient.
We suggest a first line, short-term therapy in seriously invalid patients as well as a second line therapy after IFN α and/or vinblastine failure. Further studies are required to standardize the paclitaxel treatment schedule and dosage in the disseminated classic type of KS.
Figures and Tables
 | Fig. 1(A) Clinical appearance before treatment with paclitaxel. Multiple reddish-purple plaques and patches with pitting edema distributed widely on upper and lower extremities. (B) Residual hyperpigmented patches after 2 infusions of paclitaxel. Note the edema has all resolved and nearly skin lesions are disappeared. |
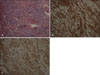 | Fig. 2(A) Skin biopsy specimen showing infiltrate of spindles shaped cells and slit-like vascular spaces containing erythrocytes in Kaposi's sarcoma (H&E stain, ×200). (B) CD31 stain showing positive reaction to well-formed flattened endothelial cell lining the vascular cleft and spindle-shaped stromal cells (×200). (C) CD34 stain showing prominent positive reaction in the spindle cell population (×200). |
References
1. Sarid R, Klepfish A, Schattner A. Virology, pathogenetic mechanisms, and associated diseases of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8). Mayo Clin Proc. 2002. 77:941–949.

2. Ensoli B, Stürzl M. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998. 9:63–83.

3. Kang MJ, Kim TY. Recurrent classical type of Kaposi's sarcoma treated by interferon-alpha. Ann Dermatol. 2008. 20:162–165.

4. Sung JC, Louie SG, Park SY. Kaposi's sarcoma: advances in tumor biology and pharmacotherapy. Pharmacotherapy. 1997. 17:670–683.
5. Fenig E, Brenner B, Rakowsky E, Lapidoth M, Katz A, Sulkes A. Classic Kaposi sarcoma: experience at Rabin Medical Center in Israel. Am J Clin Oncol. 1998. 21:498–500.
6. Rowinsky EK. Paclitaxel pharmacology and other tumor types. Semin Oncol. 1997. 24:6 Suppl 19. S19-1–S19-12.
8. Kim HO, Lee BY, Han HJ, Park CW, Lee CH, Kim JH. A case of Kaposi's sarcoma treated with paclitaxel. Korean J Dermatol. 2005. 43:1119–1123.
9. Iscovich J, Boffetta P, Winkelmann R, Brennan P, Azizi E. Classic Kaposi's sarcoma in Jews living in Israel, 1961-1989: a population-based incidence study. AIDS. 1998. 12:2067–2072.

10. Cattani P, Cerimele F, Porta D, Graffeo R, Ranno S, Marchetti S, et al. Age-specific seroprevalence of Human Herpesvirus 8 in Mediterranean regions. Clin Microbiol Infect. 2003. 9:274–279.

12. Tschachler E. Wolff K, Goldsmith LA, Kats SI, Girchrest BA, Paller AS, Leffel D, editors. Kaposi sarcoma. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGrow-Hill;1183–1187.
13. Schwartz RA, Micali G, Nasca MR, Scuderi L. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008. 59:179–206.

14. Toschi E, Sgadari C, Monini P, Barillari G, Bacigalupo I, Palladino C, et al. Treatment of Kaposi's sarcoma--an update. Anticancer Drugs. 2002. 13:977–987.

15. Brambilla L, Romanelli A, Bellinvia M, Ferrucci S, Vinci M, Boneschi V, et al. Weekly paclitaxel for advanced aggressive classic Kaposi sarcoma: experience in 17 cases. Br J Dermatol. 2008. 158:1339–1344.

16. Baskan EB, Tunali S, Adim SB, Kiyici M, Ali R. Treatment of advanced classic Kaposi's sarcoma with weekly low-dose paclitaxel therapy. Int J Dermatol. 2006. 45:1441–1443.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download