Abstract
Background
Though elastic fibers are as important as collagen fibers in interpretation of the histopathologic findings, it is impossible to observe them on the hematoxylin & eosin (H&E) stained specimen.
Methods
Normal skin tissue sections were stained in 4 different ways (unstained, hematoxylin only, eosin only, H&E) and observed under a fluorescence microscope using a FITC filter set. Fluorescent findings of 30 H&E-stained specimens showing abnormal dermal findings were compared with bright field findings of Miller's elastic stained specimen.
Results
Strong eosin fluorescence was related to the differential binding property of eosin with elastic fibers. Hematoxylin stain quenched excessive eosin fluorescence from other tissue components and contributed to better contrast. Fluorescence microscopy of H&E-stained sections was found to be especially useful in observing mature elastic fibers in the reticular dermis. In 74% of the specimens, eosin fluorescence findings of elastic fibers in reticular dermis matched well with that of specimens with elastic fiber special stain.
Since elastic fiber structure and tissue distribution are key elements for understanding physical properties of skin tissues, they are important in the development of methods to study the morphology and spatial organization of elastic fibers1,2. Elastic fibers are difficult to demonstrate in routine hematoxylin-eosin (H&E) stained specimens. But under a fluorescence microscope, routinely processed and stained elastic fibers show fluorescence without any special handling3. This may be due to the binding of eosin dye to the elastic fibers. Although eosin is usually not regarded as a fluorochrome, high-fluorescence emission has been described for this dye2,4. The aim of the present study was to analyze skin elastic fibers by characterizing the eosin fluorescence detected by fluorescence microscopy and to investigate the characteristics of eosin fluorescence in degenerative elastic fibers.
In order to investigate the origin of fluorescence from elastic fibers stained with H&E and the detailed roles of each staining agent used in H&E staining, we stained separately normal skin sections in four different ways: unstained, hematoxylin only, eosin only, and H&E. We used a microscope which enables us to use both bright field and fluorescence microscopy. A BX50 (Olympus, Tokyo, Japan) microscope operating through a reflected light fluorescence attachment (BX-FLA) was used. Fluorescence-stained sections were examined using the following excitation/emission filter combinations (filter set for FITC): 450~480 nm exciter filter and 515 nm barrier filter and 505 nm dichroic mirror. Images were obtained by digital scientific cooled CCD camera (SPOT-RT Color model, Diagnostic Instrument, Sterling Heights, MI, USA) and SPOT imaging software.
Skin specimens from thirty clinical cases with various dermal histological abnormalities, were selected for fluorescence microscopic examination of elastic fibers. Fluorescence microscopic findings were then compared with those of elastic fiber special stains to determine the relationship of the two, and to ascertain the meaning of these findings.
Bright yellowish fluorescence of elastic fibers, which was well differentiated with collagen fibers by their intensity and more greenish color was observed under a fluorescence microscope. In bright field microscopy of H&E-stained specimens, elastic fibers and collagen fibers were indistinguishable due to similarity in their pinkish color shades. Fluorescence findings for elastic fibers matched elastic fiber special stain (Miller's elastic stain) findings (Fig. 1).
Unstained sections of the same material were examined for verification of the contribution of autofluorescence of elastic fibers to the final image. Unstained tissue sections showed a very weak and dull green fluorescence pattern of elastic fibers. This finding was not strong enough for its adequate distinction from other structures (Fig. 2).
Hematoxylin stained section showed no fluorescence (Fig. 2).
Eosin stained sections showed a very bright yellowish fluorescence of elastic fibers. But collagen fibers and other components (epidermis, hair) also showed excessive intensity of yellowish to greenish fluorescence enough to hamper optimal interpretation of elastic fibers. Hence, elastic fibers appeared in less definite contrast with collagen fibers (Fig. 2).
In the H&E stained section, excessive eosin fluorescence of collagen fibers and other components observed in the eosin only stained section was decreased. Hence, elastic fibers appeared to have a more striking contrast with collagen fibers. Thus, hematoxylin stain quenched excessive eosin fluorescence and contributed to better contrast (Fig. 2).
We observed some difference in findings between the fluorescence of elastic fibers in the reticular dermis and in the papillary dermis. Mature elastic fibers in the reticular dermis expressed bright yellowish fluorescence, and could be observed by fluorescence microscope. But, the thin elastic fibers (oxytalan, elaunin fibers) in the papillary dermis showed very weak fluorescence, and hence hardly ever observed by fluorescence microscopy (Fig. 3).
Fluorescence findings of elastic fibers matched well between elastic fiber special stain and fluorescence microscopy of H&E-stained section in 74% of the specimens. Usually, the matched specimens were of elastic fibers in sun-protected skin. But different elastic fiber patterns were noticed in the remaining 26% of specimens, which indicated degeneration of elastic fibers by photo aging or other causes (Table 1).
Elastic fibers of sun exposed areas (face, neck and forearms) showed decreased fluorescence or no fluorescence. Hence, the elastic fibers of sun damaged skin can be distinguished from normal elastic fibers. But it is impossible to make this distinction using the classic elastic fiber special stain, because in that stain all elastic fibers are stained blue-black similarly (Fig. 4).
The fluorescence findings of pseudoxanthoma elasticum (PXE) showed unique mixed findings of a bright yellowish fluorescence pattern of the normal elastic fibers and a mottled fluorescence pattern of the degenerative elastic fibers with calcium deposition. It is impossible to make this distinction using the classic elastic fiber special stain because in that stain all elastic fibers and calcium are stained blue-black (Fig. 5).
Elastic fibers help the skin return to its normal configuration after being stretched or deformed. The elastic fibers consist of two components: microfibrils and matrix elastin. The microfibrillar component amounts to only 15% of the elastic fiber, whereas the amorphous, electron-lucid elastin makes up 85% of the fiber5. In light microscope sections that are routinely stained, elastic fibers are inconspicuous. With special elastic tissue stains, such as orcein or resorcin-fuchisin, or in plastic-embedded sections they are found entwined among the collagen bundles6. But without any special stain, routinely processed and H&E stained elastic fibers demonstrated fluorescence under the fluorescence microscope. The fluorescence finding was related to differential binding of eosin with tissue components rather than with auto-fluorescence of the tissue components themselves2-4.
Eosin is a very common plasmal stain. It is acidic and usually employed in combination with basic dyes such as hematoxylin2. Although eosin is usually not regarded as a fluorochrome, high-fluorescence emission has been described for this dye. Eosin showed a peak emission centered at 550 nm after being excited by light of 490 nm7. And hematoxylin stain quenched excessive eosin fluorescence and contributed to better contrast8,9.
H&E staining of elastic fibers observed under fluorescence microscopy showed bright yellowish fluorescence, which would be impossible to observe under a bright field microscope. The intensity of fluorescence of elastic fibers showed a difference between the reticular dermis and the papillary dermis. Mature elastic fibers in the reticular dermis expressed bright yellowish fluorescence, and could be observed through a fluorescence microscope. But, thin elastic fibers (oxytalan, elaunin fiber) in the papillary dermis showed almost no fluorescence, and hence hardly ever observed by fluorescence microscopy. This result is consistent with the fact that the reticular dermis has more elastic fibers than the papillary dermis. It is known that the elastic system of the dermis consists superficially of thin bundles of microfibrils, which become associated with progressively larger amounts of amorphous elastin and increase in size from the deeper papillary to the reticular layer1,5. Basically, the elastic fiber system of normal human skin consists of three types of fibers: oxytalan, elaunin, and elastic fibers, which are believed to differ in their relative contents of microfibrils and elastin. According to ultrastructural analysis, oxytalan fibers contain only microfibrils, elaunin fibers contain small quantities of amorphous elastin, and elastic fibers are predominantly elastin. It is the elastin that stains with elastic tissue stains, whereas the microfibrils are the elastic resilient component of the elastic fiber1. Hence, the mature elastic fibers which are elastin-rich showed strong fluorescence. On the other hand, thin elastic fibers (oxytalan, elaunin) that had little elastin showed only very weak or almost no fluorescence.
Fluorescence images of the H&E stained sections were adequate for morphometric evaluation for elastic fibers in the dermis. And the fluorescence findings of elastic fibers matched well between elastic fiber special stain and fluorescence microscopy of the H&E-stained specimen in 74% of the specimens. But different elastic fiber patterns were found in the remaining 26% of specimens, which demonstrated degeneration of elastic fibers by photo aging or other causes. Elastic fibers of the sun-exposed areas showed decreased fluorescence or no fluorescence. Elastic fibers are acidophilic and capable of relatively selective reactions with the acid dye (eosin)10. Sun exposure induced a change in basophilic degeneration with deposition of lysozyme in the dermal elastic fiber11. This change would weaken the bonding with the acid dye (eosin), and decrease fluorescence of the elastic fibers in photo-aged skin. For this reason, elastic fibers of sundamaged skin could be distinguished from normal elastic fibers. But it has been impossible to make this kind of distinction using the classic elastic fiber special stain method.
Sometimes, analysis of skin elastic fibers by fluorescence microscopy shows better tissue morphological details than that by special stains. PXE is an inherited multi-system disorder with primary abnormalities in the elastic fibers12. Histological changes in PXE include fragmentation, clumping and calcification of elastic fibers as well as basophilic swollen and degenerated elastic fibers in the middle and deep reticular dermis13. The fluorescence findings of PXE showed unique mixed findings of a bright yellowish fluorescence pattern in the normal elastic fibers and a mottled fluorescence pattern with calcium deposition in the degenerative elastic fibers. It would be impossible to make this distinction using the classic elastic fiber special stain, because in elastic fiber special staining, all elastic fibers and calcium stain blue-black.
The present study has some limitations. We used various skin specimens to compare findings between fluorescence microscopy of H&E stained sections and classic elastic fiber special staining. Because we only used skin specimens showing histological abnormalities of the dermis, further studies on various human skin disorders regardless of dermal abnormalities are needed to elucidate the efficacy of eosin fluorescence microscopy.
In conclusion, analysis of skin elastic fibers by fluorescence microscopy is a useful and complementary method to reveal hidden elastic fibers in H&E-stained specimens without using any additional special stains and to show better tissue morphological details than that can be achieved by special stains. Application of fluorescence microscopy on routine H&E-stained specimens may unveil more useful findings and hidden information in specimens of various skin diseases in the future.
Figures and Tables
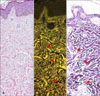 | Fig. 1Basic features of the fluorescence of elastic fibers stained with H&E. (A) Elastic fibers are invisible by bright field microscopy (H&E, ×200). (B) Elastic fibers in the same tissue specimen (arrow heads) are visible by fluorescence microscopy (brightly yellow dots and streaks) (H&E, ×200). (C) Miller's elastic stain of elastic fibers shows corresponding pattern in the dermis as observed by fluorescence microscopy (arrow head) (×200). |
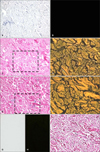 | Fig. 2Basic features of eosin fluorescence in different steps of H&E staining process. (A, B) Hematoxylin stain only, (C, D) eosin stain only, (E, F) H&E, (G, H) unstained background, (I) Miller's elastic stain. (D, F) Photographic enlarged view of dotted box area in C, E. (A, C, E, G, I) Bright field microscopic findings (×200), (B, D, F, H) fluorescence microscopic findings (×200). |
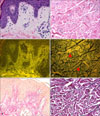 | Fig. 3Eosin fluorescence findings of elastic fibers in the dermis. (A, C, E) Elastic fibers (oxytalan and elaunin) in the papillary dermis. (B, D, F) Mature elastic fibers (arrow heads) in the reticular dermis. (A, B) Bright field microscopy (H&E, ×400), (C, D) fluorescence microscopy (H&E, ×400), (E, F) bright field microscopy (Miller's elastic stain, ×400). |
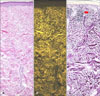 | Fig. 4Loss of fluorescence in photoaged skin. Eosin fluorescence was lost in photo damaged elastic fibers of the papillary dermis. But in Miller's elastic stain, dark clumping degenerated elastic fibers (red arrow) in the papillary dermis stained the same as normal elastic fibers in the reticular dermis. (A) Bright field microscopy (H&E, ×100), (B) fluorescence microscopy (H&E, ×100), (C) bright field microscopy (Miller's elastic stain, ×100). |
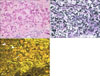 | Fig. 5Eosin fluorescence findings in pseudoxanthoma elasticum. (A) Calcified altered elastic fibers were observed (H&E, ×400). (B) Elastic fibers showed the same pattern as H&E stain (Miller's elastic stain, ×400). (C) Intact elastic fibers (arrow head), degenerative elastic fibers (thick arrow) and brown color calcium depositions (thin arrow) were observed in different pattern to H&E or elastic stain (fluorescence microscopy, ×400). |
References
1. Schwartz E, Fleischmajer R. Association of elastin with oxytalan fibers of the dermis and with extracellular microfibrils of cultured skin fibroblasts. J Histochem Cytochem. 1986. 34:1063–1068.

2. Goldstein DJ. The fluorescence of elastic fibres stained with eosin and excited by visible light. Histochem J. 1969. 1:187–198.

3. Song H, Kim C. Eosin fluorescence microscopy of hematoxylin-eosin stained histopathologic specimens [abstract]. J Invest Dermatol. 2000. 114:821.
4. Huntington H. Autofluorescence of eosinophilic substances. Arch Pathol Lab Med. 1986. 110:93.
5. Varadi DP. Studies on the chemistry and fine structure of elastic fibers from normal adult skin. J Invest Dermatol. 1972. 59:238–246.

6. Elder DE, Elenitsas R, Murphy GF, Johnson BL, Xu X, editors. Lever's histopathology of the skin. 2009. 10th ed. Philadelphia: LIppincott Williams & Wilkins;49.
7. Chadwick CS, Mc EM, Nairn RC. Fluorescent protein tracers; a trial of new fluorochromes and the development of an alternative to fluorescein. Immunology. 1958. 1:315–327.
8. de Carvalho HF, Taboga SR. Fluorescence and confocal laser scanning microscopy imaging of elastic fibers in hematoxylin-eosin stained sections. Histochem Cell Biol. 1996. 106:587–592.

9. de Carvalho HF, Taboga SR. The applicability of hematoxylin-eosin staining plus fluorescence or confocal laser scanning microscopy to the study of elastic fibers in cartilages. C R Acad Sci III. 1996. 319:991–996.
10. Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 2002. 15th ed. London: Churchill Livinstone;143.
11. Suwabe H, Serizawa A, Kajiwara H, Ohkido M, Tsutsumi Y. Degenerative processes of elastic fibers in sun-protected and sun-exposed skin: immunoelectron microscopic observation of elastin, fibrillin-1, amyloid P component, lysozyme and alpha1-antitrypsin. Pathol Int. 1999. 49:391–402.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download