Abstract
Background
Topical retinoids induce skin fragility. As corneodesmosomes are important adhesion structures in the epidermal cohesion, an effect of retinoids on corneodesmosomes has been suspected.
Objective
The aim of this study was to investigate the effect of retinoid on the expression of corneodesmosomal components including desmoglein (DSG) 1, desmocollin (DSC) 1, corneodesmosin (CDSN) and kallikrein (KLK)s.
Methods
2% all-trans-retinol or ethanol was applied to the back of hairless mice for five days, and the structure of the stratum corneum was examined by transmission electron microscopy. The cultured human keratinocytes were treated with all-trans-retinoic acid (RA) in low or high calcium media for 24 hours.
Results
Topical retinol increased corneocyte detachment and degradation of corneodesmosomes. RA significantly decreased DSG1 and DSC1 expression at the mRNA and protein levels in keratinocytes that were cultured in both low- and high-calcium media. On the other hand, CDSN mRNA levels did not decrease in low-calcium media or increase in high-calcium media after RA treatment. KLK5 and KLK7 expression did not increase after RA treatment.
Many patients undergoing topical retinoid therapy experience "retinoid dermatitis", a retinoid-specific irritant contact dermatitis, characterized by erythema, scaling, dryness, burning sensation, and pruritus1. Although clinical and histological alterations induced by topical retinoids are similar to those of nonspecific irritant-contact dermatitis, retinoid dermatitis is now accepted as distinct from nonspecific irritant-contact dermatitis in that the former is at least, in part, mediated through receptor-mediated cell signaling2,3. Among the various retinoid-induced cutaneous changes, epidermal hyperproliferation and resultant scaling have been demonstrated to be mediated by retinoic acid receptor (RAR) signaling based on the finding that topical tretinoin failed to induce a hyperplasia and desquamation response in the epidermis of genetically engineered mice with a functional deficiency of RAR4. However, it is still not clear how the interaction between retinoids and their receptors leads to retinoid dermatitis.
Scaling and increased skin fragility are well-known adverse effects of retinoid therapy. Some previous studies have shown that scaling seems to be more prominent in retinoid-treated skin, compared to skin exposed to other irritants5,6. Topically applied 0.05% or 0.1% all-trans retinoic acid (RA) appeared to cause more intense scaling than another irritant, sodium lauryl sulphate, in a 24-hour occlusive patch test5. Topical glycolic acid, another irritant, has also been shown to induce less scaling when compared with RA7. Scaling results from abnormal desquamation. The desquamation of stratum corneum (SC) is an active process of corneocyte shedding from the outer skin surface as a result of proteolytic degradation of corneodesmosomes, which are specialized structural proteins forming links or welds between adjacent corneocytes in the SC layer8. Three proteins have been described as components of the extracellular part of corneodesmosomes: the two desmosomal cadherins, desmoglein (DSG) 1 and desmocollin (DSC) 1 that associate in a calcium-dependent manner9, and corneodesmosin (CDSN), a characteristic adhesive glycoprotein secreted by the keratinocytes of granular layers and then incorporated into the desmosomes10. Degradation of corneodesmosomes is an important process during desquamation and is facilitated in a pH-dependent manner by the action of specific serine proteases in the epidermis, such as the human tissue kallikrein 5 (KLK5) and kallikrein 7 (KLK7)11,12. Corneodesmosomal proteins that have been reported to be degraded during desquamation include DSG1, DSC1, plakoglobin, and CDSN13,14.
A recent study demonstrated that the enzymatic degradation of corneodesmosomes weakened the strong interaction between corneocytes in the SC microstructure, making the tissue more susceptible to delamination fracture15. The pronounced scaling and peeling observed in retinoid dermatitis may be the indirect consequence of retinoid-induced epidermal proliferation and increased SC turnover, but RA could directly regulate the expression of corneodesmosomal components and thereby alter the desquamation process. To investigate this issue, we examined the ultrastructure of corneodesmosomes in the skin of hairless mice treated with a topical retinol application and compared the retinol-treated epidermis with vehicle-treated epidermis. In addition, to confirm whether the effects of RA on the structural alteration of corneodesmosomes were due to the ability of RA to directly regulate the expression of corneodesmosomal proteins, the effects of RA on the mRNA and protein levels of DSG1, DSC1, and CDSN (three major components of corneodesmosomes) were evaluated in cultured keratinocytes. We also measured mRNA expression levels of KLK5 and KLK7 to rule out the possibility that these two serine proteases could affect expression levels of corneodesmosomal proteins. Our results suggest that the downregulation of DSG1 and DSC1 without activation of KLKs by RA may be associated with abnormal desquamation observed in retinoid dermatitis.
Sixteen 6-week-old, female, hairless mice were used in this study. All animals were caged under controlled conditions (temperature: 23±3℃ and humidity: 50±10%). Throughout the experiment, the mice were fed on standard mouse pellets and water ad libtum. A total of 2% all-trans-retinol (Sigma-Aldrich, St. Louis, MO, USA) in ethanol was applied to the left dorsal skin region, and ethanol alone as a vehicle was applied to the right dorsal skin region. Each solution was applied as a volume of 20 µl once a day for 5 consecutive days.
Samples for electron microscopy were obtained 24 hours after the last application, incubated in a fixative containing 2% glutaraldehyde, 2% formaldehyde, 90 mM potassium oxalate and 1.4% sucrose overnight prior to post-fixation. After post-fixation, all samples were dehydrated serially in an ascending alcohol series and embedded in 100% Polybed resin. Sections were viewed and photographed with an electron microscope (H-500, Hitachi, Japan).
Human keratinocytes were isolated from normal human foreskin. The keratinocytes were seeded and maintained in 0.06 mM Ca2+ keratinocyte growth medium (Cambrex, Walkersville, MD, USA) containing epidermal growth factor (100 ng/ml), bovine pituitary extract (70µg/ml), hydrocortisone (0.5µg/ml), and insulin (5µg/ml) in an incubator at 37℃ and 5% CO2. Once the cells attached, the culture medium was switched to either 0.06 mM Ca2+ (low Ca2+) or 1.5 mM Ca2+ (high Ca2+). When keratinocytes covered more than 80% of the dish area, 10-6 M and 10-7 M all-trans-retinoic acid (RA, Sigma-Aldrich) was added to the culture media, and the cells were cultured for 24 hours.
Keratinocytes were lysed in a 500µl lysis buffer containing 1.5% SDS and 0.0625 mol/L Tris-HCl buffer at a pH of 6.8, supplemented with 5% β-mercaptoethanol, 2 mM/L phenylmethylsulphonyl fluoride (PMSF; Sigma), and 2 mM/L EDTA. The cells were gathered by cell scraper and homogenized on ice. The mixture was boiled for 4 minutes and centrifuged to obtain a supernatant. The amount of protein was then quantified with a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA) using the Bradford method. The protein extracts were loaded in 50µg-per-lane aliquots and separated with a NuPAGE gel (3-8% Novex Tris-Acetate Gel). After electrophoresis, proteins were transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Membranes were incubated with antibodies for DSG1 and DSC1 (Progen, Heidelberg, Germany), KLK5, KLK7 (Santa Cruz, CA, USA) and β-actin (Sigma) at 4℃. After overnight incubation, membranes were washed in Tris-buffered saline plus 0.1% Tween-20. Peroxidase-conjugated anti-mouse IgG (DAKO, Glostrup, Denmark) for DSG1, DSC1, or β-actin and anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA) for KLK5 and KLK7 were used as secondary antibodies. Detection was carried out using an enhanced chemiluminescence detection kit (Amersham, Little Chalfont, UK). For quantitative comparison, the blotting intensity of each band on X-ray film (Kodak, Rochester, NY, USA) was assessed with the GelDoc imaging system (BioRad) using Quantity One software (Bio-Rad). Each experiment was repeated three times.
RNA was extracted from cultured keratinocytes with the TRIzol reagent (Invitrogen). RT-PCR was performed using RNA-PCR kit version 3.0 (Takara, Otsu, Japan). Brief complementary DNA amplification conditions were as follows. Cycles of denaturing at 94℃ for 30 seconds, annealing at 60℃ for 30 seconds and extension at 72℃ for 90 seconds were repeated 35 times with a final elongation step at 72℃ for 5 minutes. PCR products were electrophoresed in 4% Nusieve 3:1 agarose gel (FMC, Rockland, ME, USA) and analyzed quantitatively with the GelDoc imaging system (BioRad). We employed β-actin as a control. Primers used were as follows: DSG1, sense 5'-cttcaacgactgttaggtatgt-3', antisense 5'-gcaagttctttgaagattatcatc-3'; DSC1, sense 5'-taaagcagcgagctcacaaa-3', antisense 5'-ctgcatccactgcaacaact-3'; CDSN, sense 5'-atgatggcactgctgctg-3', antisense 5'-aaggtgccaatgctcttagc-3'; KLK5, sense 5'-gccacactgcaggaagaaa-3', antisense 5'-ggatttgaccccctggaa-3'; KLK7, sense 5'-gcatccccgactccaagaa-3', antisense 5'-cagggtacctctgcacaccaa-3'; β-actin, sense 5'-atggatgatgatatcgccgcg-3', antisense 5'-atgtcgtcccagttggtgacgat-3'. Each experiment was repeated three times.
Compared to the ethanol-treated epidermis, the stratum corneum was considerably disrupted by treatment with 2% all-trans-retinol in ethanol for 5 days. As illustrated in Fig. 1B, the corneocytes were widely separated and many of them were removed from the upper stratum corneum, resulting in a thinning of the stratum corneum layer in retinol-applied skin compared to vehicle-applied skin. Intercellular edema and degenerated corneodesmosomes were more pronounced in retinol-applied skin compared to vehicle-applied skin (Fig. 1C, B).
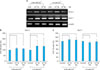
Treatment of keratinocytes with RA (10-6 M or 10-7 M) for 24 hours resulted in a statistically significant decrease in DSG1 and DSC1 mRNA levels in keratinocytes under low-calcium (0.06 mM Ca2+) and high-calcium (1.5 mM Ca2+) conditions. In contrast to DSG1 and DSC1, no effect on the transcriptional level of CDSN was observed (Fig. 2D). Moreover, RA (10-6 M or 10-7 M) increased the CDSN mRNA levels in keratinocytes cultured under high-calcium conditions (Fig. 2D).
To determine whether RA could regulate the protein levels of DSG1 and DSC1 in addition to regulating transcription, DSG1 and DSC1 protein levels were assessed by western blotting in RA-treated keratinocytes. Western blot analysis showed that the DSG1 protein significantly decreased in keratinocytes cultured with RA (10-6 M or 10-7 M) for 24 hours in both low- and high-calcium media. Quantitative results showed that 10-6 M or 10-7 M RA decreased DSG1 protein expression by about 90% in keratinocytes cultured in low-calcium media. The addition of 10-6 M RA and 10-7 M RA to cultured keratinocytes in high-calcium media for 24 hours also resulted in decreased levels of DSG1 protein by 55% and 58%, respectively (Fig. 3A, B). The protein expression of DSC1 significantly decreased by about 80% and 87% in keratinocytes treated with RA (10-6 M or 10-7 M) for 24 hours in low-calcium media, respectively. In high-calcium media, 10-6-M and 10-7-M RA also decreased the protein levels of DSG1 by 80% and 72%, respectively (Fig. 3A, C).
To verify that the inhibitory effect of RA on the expression of two corneodesmosomal components, DSG1 and DSC1, was due to the activation of the two serine proteases in the kallikrein family (KLK5 and KLK7), the mRNA and protein levels of the two serine proteases were assessed in RA-treated keratinocytes by RT-PCR and western blotting, respectively. RT-PCR showed that there was no statistically significant difference in KLK5 and KLK7 mRNA expression in keratinocytes treated with RA (10-6 M or 10-7 M) for 24 hours compared to untreated keratinocytes, under both low- and high-calcium conditions (Fig. 4). Western blot analysis also showed no significant difference in protein levels of KLK5 and KLK7 in cultured keratinocytes treated with RA (10-6 M or 10-7 M) for 24 hours in low-calcium (0.06 mM Ca2+) media compared to untreated keratinocytes. In high-calcium media (1.5 mM Ca2+), KLK5 protein levels in keratinocytes dose-dependently decreased by RA (10-6 M or 10-7 M) treatment for 24 hours, whereas KLK7 protein levels did not change following RA (10-6 M or 10-7 M) treatment (Fig. 5).
Retinol treatment on the skin of hairless mice showed increased detachment of corneocytes and degeneration of the corneodesmosomes, which was clearly apparent on electron microscopy images and consistent with the clinical findings of scaling or peeling. To elucidate the underlying mechanisms for this retinoid-induced degeneration of the corneodesmosomes, we investigated the effect of RA on the expression of the three major components of corneodesmosomes in cultured keratinocytes with different calcium concentrations.
By using RT-PCR, we found that 10-6 M and 10-7 M RA treatment significantly decreased mRNA levels of DSG1 and DSC1 in keratinocytes, as compared to the untreated control. RA also reduced DSG1 and DSC1 protein expression by more than 50%. These results are in agreement with a previous study which showed that the transcription levels of DSG1 and DSC1 were dose-dependently decreased by retinoic acid treatment in gingival epithelial cells16. Another in vivo study also demonstrated that 0.025% topical RA treatment reduced DSC1 expression, as compared to a non-specific irritant, sodium dodecyl sulphate, in human epidermis6.
It has been demonstrated that factors with a role in regulating epidermal differentiation may modulate the expression of desmosomal cadherins17,18. For example, calcium and protein kinase C regulate the mRNA expression of DSG1 and DSG3 in cultured human keratinocytes17. The Rho-activated kinase signaling pathway, which is implicated in keratinocyte differentiation, has also been shown to upregulate genes for desmosomal cadherins18. Since retinoid is well-known for modulating growth and differentiation of the epidermis, we may expect that retinoid regulates the expression of desmosomal cadherins. There have been many studies which suggest that retinoid-induced skin fragility could partly result from the alteration or disruption of epidermal desmosomes7,16,19-22. An electron microscopy study showed that systemic retinoid can cause skin fragility through a decreased number of tonofilaments and desmosomes in the epidermis7. We previously reported that RA induced loosening of the cell-to-cell contact of cultured keratinocytes and decreased the production of desmosomal proteins using immunohistochemistry and immunoblot analyses19. In previous in vitro studies, the RA-induced reduction of desmosomal cadherin expression was reported in cultured embryonic chick skin20 and in a human keratinocyte cell line (HaCaT cells)21. An in vivo study using immunohistochemistry and western blot analysis showed that while some desmosomal components including desmoplakin, DSG1, plakophilin 1, and DSC3 were reduced equally by topical retinoids or another irritants like sodium lauryl sulphate, DSC1 and perhaps DSC2 were specifically reduced in retinoid-treated skin compared to SDS-treated skin22. Retinoic acid also disintegrated desmosomes by depriving cells of DSG1, DSC1, keratin 13, and keratin 14 in stratified oral keratinocytes16. Our results support these previous findings by clearly showing that RA is able to downregulate the transcription and protein expression of DSG1 and DSC1 in vitro. However, RA did not affect the transcription levels of CDSN in keratinocytes under low-calcium media. Moreover, CDSN mRNA expression was increased by RA treatment in high-calcium media. This result was not consistent with a previous study using a cDNA macro-array, showing that RA (1µM), 9-cis retinoic acid (1µM), or all-trans-retinol (10µM) significantly decreased the gene expression of some components of desmosomes, including DSG1, DSC1, DSC3, and CDSN, in reconstituted human epidermis23. The cause of these different effects of RA on CDSN expression in cultured human keratinocytes and reconstituted human epidermis remains unknown, but it may be partially explained by the different effects of RA on cellular proliferation and differentiation depending on the state of keratinocytes. It may also be possible that CDSN expression is induced by RA, thus compensating for the loss of DSC1 and DSG1. Another possible explanation is that the different effects of RA on the expression of these corneodesmosomal components may stem from the different origins between desmosomal cadherins and CDSN. Whereas two desmosomal cadherins, DSG1 and DSC1, begin to be expressed in the suprabasal layers of the epidermis and become concentrated in the upper epidermis, CDSN is synthesized in the upper spinous and granular layer and secreted via lamellar bodies24. The increased lamellar body secretion following permeability barrier impairment by retinoid may account for the increased CDSN expression by RA. In spite of the normal or increased CDSN at transcription levels, RA-induced reduction of DSG1 and DSC1 resulted in a marked alteration of corneodesmosomal structure in our study. These findings indicate that RA-induced reduction of DSG1 and DSC1, but not CDSN, is sufficient to cause the degradation of corneodesmosomes, thereby inducing excessive scaling and desquamation, which is commonly found in retinoid dermatitis.
Desmosomal cadherins belong to the cadherin superfamily of calcium-dependent cell-cell adhesion molecules25. Four DSG (DSG 1-4) and three DSC (DSC 1-3) have been identified in the epidermis in a differentiation-specific manner26. DSG3 and DSC3 are primarily found in the basal layer, whereas DSG1 and DSC1 are found in the more differentiated upper layers27. Calcium has been implicated in the formation, assembly, and stability of desmosomes25. However, in our study, a similar reduction in DSG1 or DSC1 expression was observed in RA-treated keratinocytes under both low- and high-calcium media. These findings may suggest that although extracellular calcium could modulate the expression of desmosomes, it could not affect the ability of RA to downregulate desmosomal cadherins.
We also demonstrated that KLK5 and KLK7 expression in keratinocytes was not influenced by RA, both under low- and high-calcium media. These findings imply that KLK5 and KLK7 may not have a role in retinoid-induced desquamation processes.
Although further studies are needed to investigate the precise mechanism by which RA regulates the transcription and protein expression of corneodesmosomal components, the results presented here suggest that the significant decrease in DSG1 and DSC1 by RA can induce the degradation of corneodesmosomes within the SC independently of KLK5 and KLK7, and consequently decrease cohesiveness of the SC, thereby causing retinoid-induced desquamation.
Figures and Tables
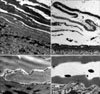 | Fig. 1Electron microscopic findings in the stratum corneum of hairless mouse skin treated with all-trans retinol. Increased corneocyte detachment and consequent thinning of the stratum corneum is clearly apparent in the all-trans retinol-treated skin (B, ×20,000) compared to the vehicle-applied skin (A, ×20,000). Degradation of the corneodesmosomes was also prominently seen in the all-trans retinol-treated skin (D, ×30,000) compared to the vehicle-applied skin (C, ×30,000). |
 | Fig. 2Effects of RA on the mRNA expression of three corneodesmosomal components (DSG 1, DSC 1, CDSN) in keratinocytes cultured in different concentrations of calcium media. Keratinocytes were treated with RA at concentrations of 10-6 M and 10-7 M for 24 hours. Total RNA was extracted and RT-PCR was performed. (A) Agarose gel electrophoresis of amplified products of DSG1, DSC1, CDSN, and β-actin cDNA. The mRNA levels of DSG 1 significantly decreased after 10-6 M or 10-7 M RA treatment compared with those of untreated cells in low- (0.06 mM Ca2+) or high-calcium (1.5 mM Ca2+) media (B). DSC1 mRNA levels also significantly decreased after 10-6 M or 10-7 M RA treatment compared with those of untreated cells in low- or high-calcium media (C). mRNA levels of CDSN was not altered after RA treatment in low-calcium media, but significantly increased in high-calcium media (D). *p<0.05; †p<0.001 (Student's t-test). RA: retinoic acid, DSG: desmoglein, DSC: desmocollin, CDSN: corneodesmosin, RT-PCR: reverse transcriptase-polymerase chain reaction. |
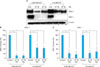 | Fig. 3Effect of RA on the protein levels of two corneodesmosomal components in keratinocytes (DSG1 and DSC1) cultured in different concentrations of calcium media. Keratinocytes were treated with RA at concentrations of 10-6 M and 10-7 M for 24 hours. Protein extraction and western blotting procedures were performed. (A) Protein expression levels of DSG1 and DSC1 were significantly decreased after 10-6 M or 10-7 M RA treatment compared with those of untreated cells in low- or high-calcium media (B, C). *p<0.001 (Student's t-test). RA: retinoic acid, DSG: desmoglein, DSC: desmocollin. |
 | Fig. 4Effect of RA on the mRNA levels of two serine proteases of the kallikrein family (KLK5 and KLK7) in keratinocytes cultured at different concentrations of calcium media. Keratinocytes were treated with RA at concentrations of 10-6 M and 10-7 M for 24 hours. Total RNA was extracted and RT-PCR was performed. (A) Agarose gel electrophoresis of amplified products of KLK5, KLK7, and β-actin cDNA. The mRNA levels of KLK5 and KLK7 did not significantly change after RA treatment in low- or high-calcium media (B, C). RA: retinoic acid, KLK: kallikrein, RT-PCR: reverse transcriptase-polymerase chain reaction. |
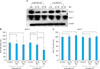 | Fig. 5Effect of RA on the protein levels of two serine proteases in the kallikrein family (KLK5 and KLK7) in keratinocytes cultured in different concentrations of calcium media. Keratinocytes were treated with RA at concentrations of 10-6 M and 10-7 M for 24 hours. Protein extraction and western blotting procedures were performed. (A) Protein expression levels of KLK5 did not significantly change after RA treatment in low-calcium media, but significantly decreased in high-calcium media (B). Protein expression levels of KLK7 did not significantly change after RA treatment in low- or high-calcium media (C). *p<0.05; †p<0.001 (Student's t-test). RA: retinoic acid, KLK: kallikrein. |
References
1. MacGregor JL, Maibach HI. The specificity of retinoid-induced irritation and its role in clinical efficacy. Exog Dermatol. 2002. 1:68–73.

2. Marks R, Hill S, Barton SP. The effects of an abrasive agent on normal skin and on photoaged skin in comparison with topical tretinoin. Br J Dermatol. 1990. 123:457–466.

3. Chen S, Ostrowski J, Whiting G, Roalsvig T, Hammer L, Currier SJ, et al. Retinoic acid receptor gamma mediates topical retinoid efficacy and irritation in animal models. J Invest Dermatol. 1995. 104:779–783.

4. Feng X, Peng ZH, Di W, Li XY, Rochette-Egly C, Chambon P, et al. Suprabasal expression of a dominant-negative RXR alpha mutant in transgenic mouse epidermis impairs regulation of gene transcription and basal keratinocyte proliferation by RAR-selective retinoids. Genes Dev. 1997. 11:59–71.

5. Effendy I, Weltfriend S, Patil S, Maibach HI. Differential irritant skin responses to topical retinoic acid and sodium lauryl sulphate: alone and in crossover design. Br J Dermatol. 1996. 134:424–430.

6. Ale SI, Laugier JP, Maibach HI. Differential irritant skin responses to tandem application of topical retinoic acid and sodium lauryl sulphate: II. Effect of time between first and second exposure. Br J Dermatol. 1997. 137:226–233.

7. Williams ML, Elias PM. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981. 117:611–619.

8. Jonca N, Guerrin M, Hadjiolova K, Caubet C, Gallinaro H, Simon M, et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J Biol Chem. 2002. 277:5024–5029.

9. Kowalczyk AP, Bornslaeger EA, Norvell SM, Palka HL, Green KJ. Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int Rev Cytol. 1999. 185:237–302.

10. Serre G, Mils V, Haftek M, Vincent C, Croute F, Réano A, et al. Identification of late differentiation antigens of human cornified epithelia, expressed in re-organized desmosomes and bound to cross-linked envelope. J Invest Dermatol. 1991. 97:1061–1072.

11. Ekholm IE, Brattsand M, Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? J Invest Dermatol. 2000. 114:56–63.
12. Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005. 124:1099–1110.

13. Simon M, Jonca N, Guerrin M, Haftek M, Bernard D, Caubet C, et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J Biol Chem. 2001. 276:20292–20299.

14. Guerrin M, Simon M, Montézin M, Haftek M, Vincent C, Serre G. Expression cloning of human corneodesmosin proves its identity with the product of the S gene and allows improved characterization of its processing during keratinocyte differentiation. J Biol Chem. 1998. 273:22640–22267.

15. Levi K, Baxter J, Meldrum H, Misra M, Pashkovski E, Dauskardt RH. Effect of corneodesmosome degradation on the intercellular delamination of human stratum corneum. J Invest Dermatol. 2008. 128:2345–2347.

16. Hatakeyama S, Hayashi S, Yoshida Y, Otsubo A, Yoshimoto K, Oikawa Y, et al. Retinoic acid disintegrated desmosomes and hemidesmosomes in stratified oral keratinocytes. J Oral Pathol Med. 2004. 33:622–628.

17. Denning MF, Guy SG, Ellerbroek SM, Norvell SM, Kowalczyk AP, Green KJ. The expression of desmoglein isoforms in cultured human keratinocytes is regulated by calcium, serum, and protein kinase C. Exp Cell Res. 1998. 239:50–59.

18. McMullan R, Lax S, Robertson VH, Radford DJ, Broad S, Watt FM, et al. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003. 13:2185–2189.

19. Kim SC, Won JH. The effects of calcium and retinoic acid on epidermal desmosomes. Korean J Dermatol. 1994. 32:820–831.
20. Peck GL, Elias PM, Wetzel B. Effects of retinoic acid on embryonic chick skin. J Invest Dermatol. 1977. 69:463–476.

21. Wanner R, Wolff B, Glowacki F, Kolde G, Wittig B. The loss of desmosomes after retinoic acid treatment results in an apparent inhibition of HaCaT keratinocyte differentiation. Arch Dermatol Res. 1999. 291:346–353.

22. Humphries JD, Parry EJ, Watson RE, Garrod DR, Griffiths CE. All-trans retinoic acid compromises desmosome expression in human epidermis. Br J Dermatol. 1998. 139:577–584.

23. Bernard FX, Pedretti N, Rosdy M, Deguercy A. Comparison of gene expression profiles in human keratinocyte monolayer cultures, reconstituted epidermis and normal human skin; transcriptional effects of retinoid treatments in reconstituted human epidermis. Exp Dermatol. 2002. 11:59–74.
24. Simon M, Bernard D, Minondo AM, Camus C, Fiat F, Corcuff P, et al. Persistence of both peripheral and non-peripheral corneodesmosomes in the upper stratum corneum of winter xerosis skin versus only peripheral in normal skin. J Invest Dermatol. 2001. 116:23–30.

25. Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007. 127:2499–2515.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download