Abstract
Background
Cutaneous adverse drug reactions (ADRs) are the most common adverse reactions attributed to drugs. A systematic and effective approach to a patient with suspected drug eruption allows for prompt recognition, classification and treatment of cutaneous ADRs. A standardized and effective approach for objective causality assessment is necessary to make consistent and accurate identification of ADRs.
Objective
Although the Naranjo algorithm is the most widely used assessment tool, it contains many components which are not suitable for clinical assessment of ADRs in Korea. The purpose of this study is to compare correlations of the Naranjo algorithm and the Korean algorithm to evaluate usefulness of both algorithms in order to make a causal link between drugs and cutaneous ADRs. In addition, this study classifies the clinical types and causative agents of cutaneous ADRs.
Methods
The authors retrospectively reviewed the clinical types and laboratory findings of patients who were diagnosed with cutaneous ADRs in the dermatology clinic at Gil hospital. One hundred forty-one patients were enrolled in this evaluation. The causal relationship of ADRs was assessed by using the Naranjo algorithm and Korean algorithm (version 2.0).
Results
A cross-tabulation analysis was applied to the Naranjo algorithm and Korean algorithm (version 2.0). Simple correlation analysis and a Bland-Altman plot were used for statistical analysis. Correlation analysis confirmed that the two assessment algorithms were significantly correlated. Exanthematous eruptions (68.8%), Stevens- Johnson syndrome (10.6%), and urticaria (8.5%) were the most common types of cutaneoues ADRs. The most common causative agents were antibiotics/antimicrobials, antipyretics/non-steroidal anti-inflammatory drugs, and central nervous system depressants.
Cutaneous adverse drug reactions (ADRs) are defined as noxious, unintended, morphologic skin changes, with or without systemic involvement, that develop after local or systemic administration of drugs in dosages commonly used for prevention, diagnosis, or treatment of diseases or modification of physiologic functions1-3. There is a wide spectrum of cutaneous ADRs, varying from transient maculopapular rashes to fatal toxic epidermal necrolysis4,5. The pattern of cutaneous ADRs and the drugs responsible for the ADRs change every year.
Most cutaneous ADRs are mild, self-limited, and usually resolve after the offending agent has been discontinued, but some severe cutaneous reactions may result in serious morbidity, and even death6-9. Since quick and careful attention to the diagnosis and treatment of cutaneous ADRs is required, a standardized approach is necessary to establish a final decision of causality to result in a consistent, accurate and reproducible identification of ADRs.
In Korea, many types of drugs are prescribed at the same time and people take oriental medicines frequently10,11. During the past few years, as more new drugs have been produced, cutaneous reactions have occurred more frequently. Further, established drugs have also been reported to cause new eruptions not previously reported10-12. This study was designed to survey the cutaneous ADRs amongst patients who visited a particular dermatologic clinic during the past three year period and to compare the accuracy of the Naranjo13 algorithm and Korean algorithm (version 2.0)14 to evaluate causal association between drugs and cutaneous ADRs.
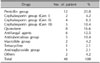
The authors examined 141 patients with cutaneous ADRs who attended the dermatology clinic at Gil hospital from January 2006 till December 2008. Mean age was 45 years (range, 1~90 years) and the majority of the patients belonged to the 41~50 year age group, followed by the 51~60 and 31~40 year age groups. Sixty-three patients (44.7%) were males and 78 patients (55.3%) were females (Table 1).
The diagnosis of cutaneous ADRs was primarily based on detailed histories and the correlation between drug intake and the onset of the ADR. The diagnosis was confirmed by observing the resolution of signs and symptoms after discontinuation of the suspected offending drugs. Medical histories and physical examinations were performed by one of the authors. A careful history of symptoms, other skin and systemic diseases, medical history, and a family history of drug eruptions or any other disease was obtained. A thorough clinical examination was carried out. The skin, hair, nails, and mucosa (eyes, oral, and genital) were examined. Complete blood cell counts, liver function tests, and renal function tests were carried out.
Each case was assessed for its causality using the Naranjo algorithm13 (Table 2) and Korean algorithm (version 2.0)14 (Table 3). The Naranjo algorithm consists of ten questions and is scored as follows: scores of 9 or 10 indicate that an event is 'definitely' an ADR, scores of 5~8 rate the likelihood of an ADR as 'probable', scores of 1~4 are 'possible' ADRs, and scores of less than 1 are 'doubtful' ADRs13. The Korean algorithm (version 2.0) consists of eight questions with scores as follows: greater than 9 is 'certain' for an ADR, 6~8 is 'probable/likely' for an ADR, 3~5 is 'possible' for an ADR, 1~2 is 'unlikely' for an ADR, and less than 0 is 'contradictory' for an ADR14.
Statistical Package for the Social Sciences (SPSS, SPSS Inc., Chicago, IL, USA) 12.0 was used for comparative analysis on the Naranjo algorithm and Korean algorithm (version 2.0). To assess the correlation between the two values, a simple correlation analysis, Pearson's simple correlation coefficient, and intra-class correlation coefficient (ICC) were used. A Bland-Altman plot was used to assess the reliability of the two values.
Cross-tabulation analysis was performed to assess the correlation and reliability of the Naranjo algorithm and Korean algorithm (version 2.0) (Table 4). Simple correlation analysis yielded a Pearson's simple correlation coefficient of 0.682 (p-value=0.0) (Fig. 1), and the measurement of inter-rater reliability by ICC was 0.67 (0.57~0.75), which ascertains a signific ant correlation of the measured quantitative values of the two assessments. A Bland- Altman plot graph was used to determine if a difference existed between the measured quantitative values of the two assessments. The analysis showed that the values were widely spread on the x-axis, revealing the existence of various test variables, which indicates tha t the test was properly conducted. In addition, most values were distributed within +/- one standard variation, suggesting that the reliability between the two assessments was high (Fig. 2). The difference in the two values became greater in inverse proportion to the average, thus low scores required more scrutiny.
The cutaneous manifestations of ADRs in the order of frequency were as follows: exanthematous eruption, 97 (68.8%); Stevens-Johnson syndrome, 15 (10.6%); urticaria, 12 (8.5%); erythema multiforme, 9 (6.4%); fixed drug eruption, 4 (2.9%); toxic epidermal necrolysis, 2 (1.4%); bullous eruption, 1 (0.7%) and pustular eruption, 1 (0.7%) (Table 5).
The interval between the time the medication was administered and the clinical appearance of the ADR was 'less than a week' in 57 cases (40.4%), 'less than 24 hours' in 38 cases (27%), and 'after four weeks' in 17 cases (5%). Thirty of 48 cases (62.5%) caused by antibiotics/antimicrobials developed ADRs within one week, and 13 cases (27.1%) developed ADRs within a day. Five cases caused by radiographic dye developed within a day.
The precise history of ADR, including allopathic, homeopathic, and herbal remedies and self-medication was obtained from patients with cutaneous ADRs, and the imputability of each drug as a possible culprit was determined. The most common causative agents were antibiotics/ antimicrobials (34.1%), followed by antipyretics/ NSAIDs (27.0%), CNS depressants (12.1%), and anticancer drugs (7.1%) (Table 6).
Among the antibiotics/antimicrobials, penicillins and cephalosporins were responsible for causing the majority of cutaneous ADRs; amoxicillin was the most common causative antibiotics (Table 7). Five cases of cutaneous ADRs were caused by iodide, which is used for computer tomography, intravenous pyelography, and myelography. The other causative agents were allopurinol, herbs, antihypertensive drugs, and gastrointestinal tract regulators/antacids (Table 6).
Liver function abnormalities, eosinophilia, and leukocytosis were present in 36.0% (31/86), 33.7% (29/86), and 15.1% of the patients (13/86), respectively. Leukocytosis was commonly present in patients with Stevens-Johnson syndrome and exanthematous eruption; liver function abnormalities were commonly present in patients with exanthematous eruptions, Stevens-Johnson syndrome, and erythema multiforme, but no cases of toxic hepatitis occurred.
The major presenting complaint was itching (61.0%), followed by fever (9.2%) and myalgia (6.4%). Other symptoms included oral mucosal lesions (5.0%), including cheilitis, stomatitis, facial edema (2.8%), and gastrointestinal symptoms, such as diarrhea and vomitting (0.7%).
Two to three percent of hospitalized medical patients are reported to have cutaneous ADRs15-17. Fatal cutaneous ADRs occur in 0.1% of medical patients and 0.01% of surgical patients15-17.
There are five reports on the incidence of cutaneous ADRs ranged from 2.6% to 8.9% assessed by dermatologist in Korea10-12,18,19. Kim and Lee12 and Bang et al.18 reported that 2.7% and 3.8% of patients visited hospitals because of cutaneous ADRs, respectively, and Shin et al.11 reported that 8.9% of inpatients had cutaneous ADRs. Hahm19 reported that 2.6% of inpatients with dermatology consultations were diagnosed with cutaneous ADRs. According to the report by Kim et al.10, the incidence of cutaneous ADRs was 1.32% in the 1970s, 1.33% in the 1980s, and 1.78% in 1990s; the gradual increase in the incidence of ADRs has been attributed to diversification, abuse, and misuse of drugs. However, the incidence and type of cutaneous ADRs evaluated by dermatologist in 2000's has not been reported on. As the incidence of ADRs is increasing, people perceive ADRs with greater concern. Proper monitoring systems and causality assessment are required to prevent the severe cutaneous ADRs which cause death, threaten lives, affect fetuses, and lead to permanent disability20,21. Recently, an ADR monitoring system (pharmacovigilance research network) has been activated in Korea. Since cutaneous ADRs are the most common, it is highly desirable for dermatologist to actively participate in the network.
The algorithms for causality assessment that are widely used in foreign nations include the Naranjo algorithm13, the French algorithm22, and the RUCAM algorithm23. These algorithms are used in foreign countries with different genetic backgrounds, investigators, and level of awareness for ADRs, and as such they might not suitable for use in Korea12,14,22. The Naranjo algorithm, which was established in 1981, is the most widely used assessment tool and consists of ten simple questions13. The merits of the Naranjo algorithm are that it is easy to apply, and assessment is possible with very little information and knowledge13,14. The downside of the Naranjo algorithm is the absence of a time-related detailed description (question 2), assessment for the response after administering a placebo that actually had not been carried out (question 6), and a non-concrete question (question 10), which may cause differences between investigators13,14,21. In fact, there was much difference between the researchers in studies undertaken to determine the causality assessment for ADRs of inpatients24,25. Similarly, the Naranjo algorithm contains many components that are not suitable for clinical assessment for ADRs in Korea and, when applied, the scores are mostly evaluated as 'probable'. Specifically, 102 cases (72.3%) were assessed as 'probable' in the current study.
Ultimately, Hong et al.14 developed the Korean algorithm, and improved ambiguous descriptions of the clinical course by adding proportional dose-dependent responses, event abatement, and clinical appearance on drug removal14,21,23. Some questions were specialized for consistent assessment as the period between administration and symptom onset, risk factors, and drug-unrelated factors14. Finally, the Korean algorithm (version 2.0) was developed as the assessment of the causal association between the suspected drug and the cutaneous ADR, which can be properly applied to Koreans. The Korean algorithms (version 2.0) will be helpful for clinical use as items with a low response rate in Korea were excluded.
The correlation and reliability of the Naranjo and Korean algorithms (version 2.0) were assessed and analyzed in this study and it was found that the Korean algorithm (version 2.0) was significantly correlated with the Naranjo algorithm. The result of cross-tabulation analysis showed that 102 cases which were assessed as 'probable' based on the Naranjo algorithm were separated into 'probable/likely' and 'certain', and 39 cases assessed as 'possible' were separated into 'possible', 'probable/likely', and 'certain' (Table 4). Thus, these findings indicate that the Korean algorithm (version 2.0) can be used more properly in ascertaining risk factors earlier and reflecting prognosis.
There was no significant difference in the incidence of ADRs between men and women, which is in agreement with the results of the other studies (Table 8). ADRs increase with age, which may be due to the increased use of medications by the elderly, the increased potential for drug-drug interactions, and altered drug metabolism by the body. The majority of the patients belonged to the 41~50 year age group in our study, the 21~30 year age group in the studies by Kim and Lee12 and Shin et al.11, and the 31~40 year age group in a study by Kim et al.10, respectively (Table 8).
The difference in various studies may be related to the regional variation in the health care-seeking behavior of the population.
The clinical types of cutaneous ADRs are classified into exanthematous eruption, urticaria, photosensitivity reaction, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, fixed drug eruption, purpura, lichenoid eruption, and bullous eruption1-3.
Of patients with ADRs, Kim et al.10 reported exanthematous eruptions in 49.3%, fixed drug eruptions in 30.1%, and urticaria in 10.1% as the most common patterns of eruptions. Of the various types of cutaneous ADRs seen in the current study, exthanthematous eruptions were the most common drug eruptions (68.8%), followed by Stevens-Johnson syndrome (10.6%) and urticaria (8.50%). The most common latent period for cutaneous ADRs was 1~7 days, which corresponds with the symptom onset of exanthematous eruption, the most common clinical type of ADR.
The frequency of clinical types and causative agents were determined for outpatients by Kim and Lee12 between 1969 and 1975, for inpatients by Shin et al.11 between 1976 and 1985, and for outpatients and inpatients by Kim et al.10 between 1989 and 1997. When compared to the present study, the most common causative agent for ADRs was salicylate in a study by Kim and Lee12, ampicillin in a study by Shin et al.11, amoxicillin in a study by Kim et al.10, and amoxicillin in the present study (Table 8).
In the order of frequency of clinical types of ADR, there were differences between the reports (Table 8). The incidence of life-threatening cutaneous ADRs, such as Stevens-Johnson syndrome, was higher compared to studies reported in Korea and internationally, which may represent that those patients with severe reactions visited the hospital in question more often. Allopurinol was a common cause of Stevens-Johnson syndrome in this survey.
Leukocytosis, eosinophilia and liver function abnormalities are often observed in patients with ADRs26-28. Of patients with ADRs, Shin et al.11 reported 42.0% had leukocytosis, 29.5% had eosinophilia, and 25.3% had liver function abnormalities; the corresponding abnormalities in laboratory findings in the present study were 15.1%, 33.7%, and 36.0%, respectively.
The Korean algorithm (version 2.0) was significantly correlated with the Naranjo algorithm and reflects prognosis properly when assessing Korean cutaneous ADRs. The findings of this study indicate that the Korean algorithm (version 2.0) can be used more properly than the Naranjo algorithm in ascertaining risk factors earlier and reflecting prognosis. Objective and consistent causality assessment is continuously required to detect the correlation of clinical index and cutaneous ADRs.
The clinical spectrum and causative agents of ADRs were similar to studies reported in Korea and internationally with minor variations. A wide clinical spectrum of cutaneous ADRs, ranging from mild maculopapular rash to serious Stevens-Johnson syndrome and toxic epidermal necrolysis were observed. The most common reaction pattern was an exanthematous eruption and the most frequent eliciting drug group was the antibiotics. Present findings on the most common reactions and eliciting drugs are in agreement with previous reports.
Figures and Tables
Fig. 1
The measured quantity values of the Korean algorithm (version 2.0) in the x-axis and the Naranjo algorithm in the y-axis are significantly correlated.
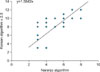
Fig. 2
The x-axis indicates the average of the two values, and the y-axis indicates the difference of the two values, being diffuse sideward means there are many experimental variables. The up-and-down lines in the graph mean a +/-1 standard variation, and the more the measured quantity values are within the two lines, the higher the reliability.

References
1. Shear NH, Knowles SR, Shapiro L. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Cutaneous reactions to drugs. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;355–362.
2. James WD, Berger TG, Elston DM. Andrews' disease of the skin. 2006. 10th ed. Philadelphia: WB Saunders;115–138.
3. Knowles SR, Shear NH. Wolverton SE, editor. Cutaneous drug reactions with systemic features. Comprehensive dermatologic drug therapy. 2007. 2nd ed. Indianapolis: Saunders Elsevier;977–988.

4. Revuz J, Valeyrie-Allanore . Bolognia J, Jorizzo J, Rapini R, editors. Drug reaction. Dermatology. 2003. 1st ed. Philadelphia: Mosby;333–353.
5. Kwon KS, Kim BS, Jang BS, Kim MB, Oh CK, Kwon YW, et al. Clinical study of drug rash with eosinophilia and systemic symptoms (DRESS) on drug eruption patients over the last 10 years (1995~2004). Korean J Dermatol. 2005. 43:1164–1169.
6. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994. 331:1272–1285.

7. Bachot N, Roujeau JC. Differential diagnosis of severe cutaneous drug eruptions. Am J Clin Dermatol. 2003. 4:561–572.

9. Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995. 155:1949–1956.

10. Kim KJ, Jeong MC, Yoo JH. Clinical study and skin tests of patients with drug eruption. Korean J Dermatol. 1998. 36:887–896.
11. Shin KS, Cho KH, Lee YS. Clinical study of hospitalized patients with drug eruption during a 10-year reriod (1975-1985). Korean J Dermatol. 1987. 25:176–182.
12. Kim SN, Lee BH. Clinical study for drug eruptions. Korean J Dermatol. 1978. 16:377–381.
13. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981. 30:239–245.

14. Hong KS, Park BJ, Sin SG, Yang JS, Lee SM, Kim YN, et al. Development of a Korean algorithm for causality assessment of adverse drug reactions. J Korean Soc Clin Pharmacol Ther. 2002. 10:129–142.

15. Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf. 2008. 31:545–556.

16. Davies EC, Green CF, Mottram DR, Pirmohamed M. Adverse drug reactions in hospitals: a narrative review. Curr Drug Saf. 2007. 2:79–87.

17. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998. 279:1200–1205.

18. Bang DS, Cho CK, Lee SN. Statistical study of dermatoses during the last 5 years (1976~1980). Korean J Dermatol. 1983. 21:37–44.
19. Hahm JH. A comparative study on clinical evaluation of inpatient dermatology consultation between 1976 and 1991. Korean J Dermatol. 1992. 30:651–658.
20. Becquemont L. Pharmacogenomics of adverse drug reactions: practical applications and perspectives. Pharmacogenomics. 2009. 10:961–969.

21. Lee SM, Hahn SK, Park BJ. Signal detection and causality evaluation for pharmacovigilance. J Korean Soc Clin Pharmacol Ther. 2005. 13:121–133.

22. Moore N, Biour M, Paux G, Loupi E, Begaud B, Boismare F, et al. Adverse drug reaction monitoring: doing it the French way. Lancet. 1985. 2:1056–1058.

23. Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993. 46:1323–1330.

24. Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991. 266:2847–2851.

25. Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Schneider HT, et al. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf. 2000. 22:161–168.
27. Gerson D, Sriganeshan V, Alexis JB. Cutaneous drug eruptions: a 5-year experience. J Am Acad Dermatol. 2008. 59:995–999.

28. Gendernalik SB, Galeckas KJ. Fixed drug eruptions: a case report and review of the literature. Cutis. 2009. 84:215–219.




 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download