Abstract
Background
Autologous platelet-rich plasma has attracted attention in various medical fields recently, including orthopedic, plastic, and dental surgeries and dermatology for its wound healing ability. Further, it has been used clinically in mesotherapy for skin rejuvenation.
Objective
In this study, the effects of activated platelet-rich plasma (aPRP) and activated platelet-poor plasma (aPPP) have been investigated on the remodelling of the extracellular matrix, a process that requires activation of dermal fibroblasts, which is essential for rejuvenation of aged skin.
Methods
Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared using a double-spin method and then activated with thrombin and calcium chloride. The proliferative effects of aPRP and aPPP were measured by [3H]thymidine incorporation assay, and their effects on matrix protein synthesis were assessed by quantifying levels of procollagen type I carboxy-terminal peptide (PIP) by enzyme-linked immunosorbent assay (ELISA). The production of collagen and matrix metalloproteinases (MMP) was studied by Western blotting and reverse transcriptase-polymerase chain reaction.
Results
Platelet numbers in PRP increased to 9.4-fold over baseline values. aPRP and aPPP both stimulated cell proliferation, with peak proliferation occurring in cells grown in 5% aPRP. Levels of PIP were highest in cells grown in the presence of 5% aPRP. Additionally, aPRP and aPPP increased the expression of type I collagen, MMP-1 protein, and mRNA in human dermal fibroblasts.
Platelet-rich plasma (PRP) is an autologous preparation of platelets in concentrated plasma. Although the optimal PRP platelet concentration is unclear, the current methods by which PRP is prepared is reported to involve 300~700% enrichment, with platelet concentrations consequently increasing to greater than 1,000,000 platelets/µl1,2. Various growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF), are secreted from the α-granules of concentrated platelets activated by aggregation inducers3. These factors are known to regulate processes including cell migration, attachment, proliferation and differentiation, and promote extracellular matrix (ECM) accumulation by binding to specific cell surface receptors4,5.
Due to the presence of high concentrations of these growth factors, PRP has been used in a wide variety of surgical procedures and clinical treatments, including the treatment of problematic wounds6 and maxillofacial bone defects7, cosmetic surgeries8,9, and gastrointestinal surgeries10. Recently, PRP has attracted attention in the field of dermatology, specifically in the aesthetic field for skin rejuvenation.
Aging of human skin results from a combination of a gradual decline in function over time (intrinsic aging) and cumulative damage caused by environmental factors (extrinsic aging), which include smoking, exposure to chemicals, and notably, ultraviolet B (UVB) radiation. In dermis, UVB has shown to stimulate collagenase production by human dermal fibroblasts (HDF) and induce collagenase gene expression11-14. In skin, which is continually exposed to UVB, collagen degeneration and altered deposition of elastic tissue result in the impairment of the structural integrity of the dermal ECM causing the skin to wrinkle. The skin's resilience is also reduced.
Topical application of growth factors stimulates the rejuvenation of photoaged facial skin, improving its clinical appearance and inducing new collagen synthesis15,16. Since PRP secretes various growth factors with roles in skin regeneration, it may be hypothesized that PRP may induce the synthesis of collagen and other matrix components by stimulating the activation of fibroblasts, thus, rejuvenating the skin. Though PRP is widely used in clinical dermatology, experimental studies confirming the effects of PRP on aged fibroblasts are very limited.
The aim of this study was to evaluate the effects of PRP on fibroblast function in vitro and remodelling of ECM components in a manner that can contribute to rejuvenation of skin, and further to generate data relevant to the potential clinical application of PRP as an anti-aging therapy.
Normal human skin samples were obtained from circumcisions, in accordance with the ethical committee approval process of Chungnam National University Hospital. Specimens were briefly sterilized in 70% ethanol, minced, and incubated in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. Dermal fibroblasts normally outgrew from the explants after 5~7 days. At confluence, cells were routinely passaged using a 1:4 split ratio. Cells were starved of serum for 24 h, then treated with activated PRP (aPRP) and activated PPP (aPPP), in serum-free medium.
PRP and PPP were prepared using a double-spin method as described in a previous study16. In brief, blood was obtained from healthy adult volunteers (n=10) after receiving informed consent and collected into tubes containing acid-citrate-dextrose solution formula A (1:4 vol/vol) anticoagulant. The citrated blood was centrifuged in a standard laboratory centrifuge for 7 min at 3,000 rpm. Subsequently, the yellow plasma (containing buffy coat with platelets and leukocytes) was taken up using a micropipette. A second round of centrifugation was performed for 5 min at 4,000 rpm. The pellet containing platelets accumulated at the bottom and the platelet-poor plasma surfaced to the top. The plasma supernatant was used as PPP and the thrombocyte pellet in 1.0 ml of plasma was used as PRP. A 1:1 (vol/vol) mixture of 0.5 M calcium chloride and thrombin was prepared in advance as an activator. A 10:1 (vol/vol) mixture of PRP or PPP and activator was incubated for 10 min at room temperature, and this mixture was regarded as aPRP or aPPP. aPRP and aPPP were centrifuged at 13,000 rpm for 15 minutes. The supernatant was stored at -0℃ until used.
Dermal fibroblasts were seeded at a density of 1×104 cells/well in six-well culture plates. After starvation for 24 h, the cells were cultured in serum-free DMEM supplemented with 0% (control), 5%, 10% aPRP or aPPP for 3 and 5 days. In parallel, the cells were cultured in DMEM supplemented with 5% FBS as positive control. To measure the proliferative potential of cells, [3H]thymidine incorporation assay was carried out as previously reported17. Cells were grown in six-well plates for 48 h, then incubated with 1µCi of [3H]thymidine for the indicated time points. After incubation, cells were washed twice with cold phosphate buffered saline (PBS), then dissolved in 0.2 M NaOH for 30 min. Lysates were then mixed with scintillation cocktail and the incorporated [3H]thymidine level was measured by liquid scintillation counting (Beckman, Mountain View, CA, USA).
Cells (1×105) were seeded in a six-well culture plate and grown to confluence in DMEM supplemented with 10% FBS. Cells were starved of serum for 24 h, then further cultured in 3 ml of fresh serum-free DMEM with aPRP and aPPP at the indicated concentrations for 2 days. The level of Procollagen type I carboxy-terminal peptide (PIP) in the conditioned media was quantified using an ELISA kit (Takara Bio Inc., Shiga, Japan) according to the manufacturer's recommended protocol. The detection limit of the assays was 10 ng/ml. Measurements were taken at least three times.
RT-PCR was performed using an RNA PCR kit (Intron Inc. Seongnam, Korea) according to the manufacturer's instructions. To summarize, 2µg of the total RNA were reverse transcribed using oligo (dT) primer. Aliquots of ReT mixture were subjected to PCR cycles with specific primer sets for type I collagen alpha 1 (COL1A1) (5'-CCTTTCTGC TCCTTTCTCCA and 5'-AGCAACACAGTTvACACAAGG), type I collagen alpha2 (COL1A2) (5'-CCCTTGTTACCG CTCTCTOC and 5'- CCGTTGGACCTCCTGG TAAT), PCR fragments were electrophoresed on 1.0% agarose gels and subsequently visualized by ethidium bromide staining.
Cells (1×106) were seeded on 100-mm culture dishes and grown to confluence. Cells were starved of serum for 24 h, then treated with aPRP and aPPP in serum-free medium for 2 days. Cell extracts were prepared in lysis buffer containing 62.5 mM Tris, pH 6.8, 50 mM DTT, 2% SDS, 10% glycerol, and proteinase inhibitors. Total protein was measured using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples were run on 10% SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, and incubated with anti-COL1 1 and COL1 2 antibodies or anti-MMP-1 and anti-MMP-3 antibodies at a dilution of 1:1000 in 5% skim milk in TBS-T (Tris Buffered Saline Tween, 25 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH=8.0). Blots were then incubated with peroxidase-conjugated secondary at a dilution of 1:2,000 in 5% skim milk in TBS-T, and were developed by enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
Statistical analysis was performed with the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) for Windows. The t-test and parametric analysis of variance (ANOVA) with Scheffe's post-hoc multiple comparison tests were used for comparison between groups with values of p<0.05 being regarded as significant. Data are presented as mean±SD.
PRP and PPP smears were stained with Wright-Giemsa-solution. Subsequent microscopic examinations indicated that PRP has a higher platelet concentration than PPP (Fig. 1A). Mean platelet counts in whole blood, PRP, and PPP were 1.8×105, 1.7×106, and 3.1×104 cells/µl, respectively. The concentration of platelets in PRP was approximately 9.4-fold greater than in whole blood (Fig. 1B).
Compared to the serum-treated control group, aPRP and aPPP showed marked increase in fibroblast proliferation. Proliferation of cells grown in medium supplemented with 5% aPRP peaked on day five (Fig. 2). Cells exposed to 5 or 10% aPRP also exhibited significantly higher rates of proliferation than positive control cells (grown in medium supplemented with 5% FBS). These results indicate that aPRP and aPPP promote the proliferation of fibroblasts, with 5% aPRP producing maximal stimulation of proliferation.
To investigate whether aPPP or aPRP regulates collagen production in cultured HDF, the PIP production was examined, which indirectly reflects overall collagen I levels. Levels of PIP showed maximum increase in cells cultured in a medium treated with 5% aPRP (Fig. 3). While aPPP increased PIP levels dose-dependently, 5% aPRP tended to induce the production of PIP more strongly than 10% aPRP. The data was consistent with the fibroblast pcroliferation assay, where the fibroblast proliferation was maximum in 5% aPRP. aPRP and aPPP both increased PIP production relative to the control, aPRP more potently than aPPP.
The effects of aPPP or aPRP were investigated on the expression of type I collagen mRNA and protein in cultured HDF. Type I collagen is the most abundant collagen component of the dermis and is assembled with pro-alpha collagen chains encoded by collagen type I alpha 1 (COL1A1), which is the major component of type Icollagen, and collagen type I alpha 2 (COL1A2).
COL1A1 and COL1A2 mRNA and prot ein expression were quantified by RT-PCR and western blotting after 48 h incubation with 5% aPRP or aPPP. Both, aPRP and aPPP stimulated the mRNA expression of the pro-alpha 1(I) and pro-alpha 2(I) chains of type I collagen more significantly than the serum-free treated control (Fig. 4A and C).
The protein expression of the COL1A1 and COL1A2 of type I collagen was significantly higher in the aPRP or aPPP treated dermal fibroblasts than the serum-free (control) treated sample (Fig. 4B and D). Though the expression of COL1A1 and COL1A2 protein were highest in dermal fibroblasts treated with 5% FBS (positive control), there was no statistical significance.
We examined the effects of aPPP or aPRP on MMP-1 and MMP-3 protein expression in cultured HDF. The fibroblasts were cultured in serum-free media for 24 h and then treated with either 5% FBS, aPPP, or aPRP. The cells were harvested after 48 h to measure the protein expression. Both aPPP and aPRP increased the expression of MMP-1 and MMP-3 proteins in HDF. The production of MMP-1 protein was highest in 5% aPRP treated fibroblasts (Fig. 5A). MMP-3 protein levels were significantly higher in 5% aPPP than in the serum-free treated fibroblast, but there was no statistical significance between 5% aPPP and 5% aPRP treated fibroblasts (Fig. 5B).
Dermal fibroblasts play a key role in the aging processes through their interactions with keratinocytes, adipocytes, and mast cells. They are also the source of ECM proteins, glycoproteins, adhesive molecules, and various cytokines18. By producing these molecules and supporting cell to cell interactions, skin fibroblasts contribute to the fibroblast-keratinocyte-endothelium axis that maintains skin integrity and youthfulness19. Conventional anti-aging strategies, such as those involving lasers and topical treatments, typically aim to increase ECM synthesis through the activation of fibroblasts. PRP is widely applied in many clinical fields of surgery for its ability to stimulate wound healing and minimize bleeding during surgery. Recently, PRP has drawn the attention of dermatologists for its various growth factors in the α-granules, including PDGF, TGF, VEGF, and IGF, ability to stimulate HDF, and treat skin wrinkles and rejuvenate the skin. However, little is known about the effects of PRP and PPP on ECM remodelling or fibroblast activation.
PRP and PPP were prepared by the manual separation of blood cell layers using a double-spin method. The activation of platelets through coagulation triggers the secretion of various growth factors, which produce mitogenic effects in various cell types20-28. The proliferation of osteoblasts26 and alveolar bone cells27 treated with 1~5% PRP was higher than that of cells treated with higher concentrations of PRP. Similarly, it was shown that maximal stimulation of HDF proliferation was achieved using 5% aPRP29. Our study indicates that aPRP stimulates the proliferation of HDF, consistent with the findings described above. Additionally, we confirmed that 5% (vol/vol) was a suitable concentration of aPRP for the treatment of HDF in vitro.
PIP production, which reflects overall collagen levels, was also highest in cells treated with 5% aPRP, the same concentration of PRP that induced maximum cellular proliferation. On the other hand, the expression of the α1 and α2 chains of type I collagen was increased in cells treated with 5% aPRP or aPPP (compared with negative control), as assessed by Western blotting. Similar induction of COL1A1 and COL1A2 mRNA expression was detected by RT-PCR, suggesting that PRP and PPP both showed an ability to induce collagen production by HDF. The reason for aPRP not showing superior efficacy on collagen production compared to aPPP is not known, but there could be several hypotheses to explain the reason. First, the platelets could secrete various growth factors from the α-granule without the activation of platelets with calcium and thrombin during the process of creating PRP. Second, although several growth factors are secreted from the platelets, its effect on the production of collagen from HDF is minimal and there is no difference among aPPP, aPRP, and serum in the production of collagen. Third, since aPPP showed increased PIP and collagen production in HDF, there may be other factors in the plasma that stimulate collagen production other than growth factors secreted from α-granules of platelets. None of the hypotheses could be proved in this study, but more investigative studies need to be done to confirm the factors stimulating the collagen production in PRP and PPP. This stimulation of de novo collagen synthesis may compensate for the defects that arise due to the fragmentation or loss of collagen in photoaged and aged skin. Accumulation of this newly-synthesized collagen may improve the structural integrity of the dermal ECM and stimulate fibroblasts to produce more collagen29.
aPRP and aPPP showed an increase in the expression of MMP-1 and MMP-3 protein. MMPs digesting various structural components of the ECM are centrally involved in dermal remodelling30. Similar induction of MMP-1 in photoaged skin may facilitate the removal of collagen fragments that damage the dermal matrix tissue, thus providing a better foundation for the deposition of new collagen29.
aPRP may induce ECM remodelling by stimulating the removal of photodamaged ECM components and inducing the synthesis of new collagen by fibroblasts, which are in turn proliferated by their stimulation. In recent studies, injection of PRP in the face and neck for revitalization obtained good results30. However, there is yet no clearly defined method for the clinical application of PRP. Methods being tested include topical application or direct injection into the skin. Strategies for increasing skin remodelling by increasing penetration and inducing mild inflammatory reactions, include the use of microneedles and lasers. However, the anti-aging effects of these plasma treatments in vivo remain to be determined. Additional studies are required to determine whether such approaches produce beneficial effects in aged skin.
To summarize, the stimulation of dermal fibroblast proliferation by aPRP was demonstrated in this study. aPPP and aPRP increased type I collagen and MMPs gene expression, suggesting that aPRP and aPPP may have the potential to promote the remodelling of aged and photoaged skin. Considering limited studies on clinical efficacy and safety, further studies are required to investigate the mechanism and safety on autologous blood-derived PRP and PPP before clinical application.
Figures and Tables
 | Fig. 1Platelet concentrations after double-spin method (n=5). (A) Microscopic examination of stained platelet smears (×40). (B) Quantified platelet counts indicate increased number of platelets in platelet-rich plasma. *p<0.05 vs. Control. nPPP: non-activated platelet-poor plasma, nPRP: non-activated platelet-rich plasma. |
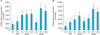 | Fig. 2aPPP and aPRP treatment increased the proliferation of cultured fibroblast. After serum-starvation for 24 h, fibroblasts were cultured with FBS, aPPP, or aPRP at concentrations as indicated. [3H]thymidine incorporation was measured at 3 and 5 days after the addition of various concentrations of aPRP and aPPP to the growth medium. (A) HDF proliferation on day 3 after control (serum-free medium), 5% FBS, aPPP, and aPRP additions. (B) HDF proliferation on day 5 after control (serum-free medium), 5% FBS, aPPP, and aPRP additions. *p<0.05 vs. control, †p<0.05 vs. 5% FBS (positive control). Con: control (serum-free medium), aPPP: activated platelet-poor plasma, aPRP: activated platelet-rich plasma, HDF: human dermal fibroblasts, FBS: fetal bovine serum. |
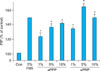 | Fig. 3Procollagen type I carboxy-terminal peptide (PIP) production in aPRP and aPPP treated human dermal fibroblasts. After 24 h of serum-starvation, cells were cultured in serum-free (Con), 5% FBS or 1, 5, or 10% aPRP or aPPP treated culture medium for 48 h. PIP was quantified using an ELISA kit. *p <0.05 vs. control, Con: control (serum-free medium), aPPP: activated platelet-poor plasma, aPRP: activated platelet-rich plasma, FBS: fetal bovine serum. |
 | Fig. 4aPRP and aPPP treatment in culture medium increased the expression of collagen type I, alpha1 and collagen type I, alpha2 mRNA and protein expression in cultured human dermal fibroblasts. After 24 h of serum-starvation, cells were cultured in serum-free (Con), 5% FBS, 5% aPRP, or aPPP treated culture medium for 48 h. α1 chains of type I collagen mRNA and protein expression were determined by (A) RT-PCR, (B) western blotting, respectively. (C) α2 chains of type I collagen mRNA expression was determined by RT-PCR, (D) western blotting. *p<0.05 vs. control, †p<0.05 vs. 5% FBS (positive control). Con: control (serum-free medium), aPPP: activated platelet-poor plasma, aPRP: activated platelet-rich plasma, RT-PCR: reverse transcriptase-polymerase chain reaction, FBS: fetal bovine serum. |
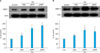 | Fig. 5aPRP and aPPP treatment in culture medium increased the expression of MMP-1 and MMP-3 protein expression in cultured HDF. (A) After 24 h of serum-starvation, cells were cultured in serum-free (Con), 5% FBS, 5% aPRP, or aPPP treated culture medium for 48 h. MMP-1 protein expression was detected by western blotting. (B) After 24 h of serum-starvation, cells were cultured in serum-free (Con), 5% FBS, 5% aPRP, or aPPP treated culture medium for 48 h. MMP-3 protein expression was detected by western blotting. *p<0.05 vs. control, †p<0.05 vs. 5% FBS (positive control). Con: control (serum-free medium), aPPP: activated platelet-poor plasma, aPRP: activated platelet-rich plasma, MMP: matrix metalloproteinases, HDF: human dermal fibroblasts, FBS: fetal bovine serum. |
References
1. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of plateletrich plasma gel preparation. J Oral Maxillofac Surg. 2000. 58:297–300.

2. Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002. 17:184–190.
3. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004. 62:489–496.

4. Freymiller EG. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004. 62:1046.

5. Wrotniak M, Bielecki T, Gaździk TS. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop Traumatol Rehabil. 2007. 9:227–238.
6. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2001. 24:483–488.

7. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:638–646.
8. Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002. 18:27–33.

9. Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous plateletpoor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001. 107:229–237.

10. Yol S, Tekin A, Yilmaz H, Küçükkartallar T, Esen H, Caglayan O, et al. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008. 146:190–194.

11. Bernstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, et al. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol. 1996. 34:209–218.
12. Petersen MJ, Hansen C, Craig S. Ultraviolet A irradiation stimulates collagenase production in cultured human fibroblasts. J Invest Dermatol. 1992. 99:440–444.

13. Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodam-aged adult human skin. J Invest Dermatol. 1995. 105:285–290.

14. Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003. 5:25–34.

15. Cameron K. Review: chemotherapy plus supportive care improves survival and quality of life in advanced or metastatic gastrointestinal cancer more than supportive care alone. Evid Based Nurs. 2005. 8:18.

16. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of plateletrich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008. 122:1352–1360.

17. Lee YS, Sohn KC, Jang S, Lee Y, Hwang C, Kim KH, et al. Anti-apoptotic role of S100A8 in X-ray irradiated keratinocytes. J Dermatol Sci. 2008. 51:11–18.

18. Le Pillouer-Prost A. Fibroblasts: whats new in cellular biology? J Cosmet Laser Ther. 2003. 5:232–238.

19. Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009. 53:96–102.

20. Gruber R, Karreth F, Frommlet F, Fischer MB, Watzek G. Platelets are mitogenic for periosteum-derived cells. J Orthop Res. 2003. 21:941–948.

21. Oprea WE, Karp JM, Hosseini MM, Davies JE. Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac Surg. 2003. 14:292–300.

22. Kocaoemer A, Kern S, Klüter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007. 25:1270–1278.

23. Kilian O, Flesch I, Wenisch S, Taborski B, Jork A, Schnettler R, et al. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res. 2004. 9:337–344.
24. Soffer E, Ouhayoun JP, Dosquet C, Meunier A, Anagnostou F. Effects of platelet lysates on select bone cell functions. Clin Oral Implants Res. 2004. 15:581–588.

25. Gruber R, Karreth F, Frommlet F, Fischer MB, Watzek G. Platelets are mitogenic for periosteum-derived cells. J Orthop Res. 2003. 21:941–948.

26. Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg. 2005. 34:420–424.

27. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006. 17:212–219.

28. Karimipour DJ, Rittié L, Hammerberg C, Min VK, Voorhees JJ, Orringer JS, et al. Molecular analysis of aggressive microdermabrasion in photoaged skin. Arch Dermatol. 2009. 145:1114–1122.

29. Almeida Issa MC, Piñeiro-Maceira J, Farias RE, Pureza M, Raggio Luiz R, Manela-Azulay M. Immunohistochemical expression of matrix metalloproteinases in photodamaged skin by photodynamic therapy. Br J Dermatol. 2009. 161:647–653.

30. Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010. 9:466–472.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download