Abstract
Cutaneous bronchogenic cysts are rare, and stem from developmental abnormalities of the tracheobronchial tree. The condition is often misdiagnosed clinically, with the correct diagnosis usually established by histopathologic examination. Published reports of bronchogenic or branchial anomalies are increasing, and the traditional defining characteristics of location and histopathology are proving to be less reliable for the identification of cutaneous bronchogenic cysts. In this report, we describe a case of a cutaneous bronchogenic cyst that presented with unusual histologic features, and was associated with several lymphoid follicles.
Cutaneous bronchogenic cysts are rare lesions that stem from developmental abnormalities of the tracheobronchial tree1. They are often misdiagnosed clinically, and the correct diagnosis usually requires histopathologic examination. Distinguishing branchial from bronchogenic cysts is particularly difficult. Zvulunov et al.2 reported that the presence of lymphoid tissue in cervical branchial cysts, and the type of epithelial lining, were the most important histologic features for distinguishing between cervical branchial cysts and cutaneous bronchogenic cysts. Although most distinctions are based on histopathology, significant overlap of pathologic findings has been noted in branchial and bronchogenic cysts3-5. Beyer et al.6 reported a case of a patient with a presternal bronchogenic sinus containing lymphoid aggregates with a germinal center. In this report, we describe an interesting case of a patient with a cutaneous bronchogenic cyst that presented with the unusual histologic feature of several lymphoid follicles.
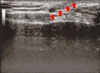
A 41-year-old male presented with a midline suprasternal nodule first noted 3 months earlier. He reported intermittent, yellowish, malodorous, secretions through a suprasternal punctum. Clinically, a 1.0×1.0 cm-sized, mobile, skin-colored, cystic mass was noted on the suprasternal notch (Fig. 1). Physical examination also revealed a small punctum in the suprasternal notch, without active drainage or signs of infection. Ultrasound examination showed a well-demarcated hypoechoic cystic mass that was approximately 1.0×1.0 cm in size (Fig. 2). No other abnormalities were found during the general physical and laboratory examination of the patient.
Total surgical excision of the skin lesion was performed under local anesthesia. There was no evidence of either extension or connections to other structures or to deep tissues. Microscopic examination revealed a cystic structure lined with ciliated, pseudostratified, columnar epithelium in the deep dermis and subcutis (Fig. 3A). Several well-circumscribed aggregations of basophilic lymphoid follicles were found in the connective tissue surrounding the cystic cavity (Fig. 3B). Goblet cells that stained positive for PAS were scattered among the ciliated epithelial cells (Fig. 3C, D). Neither smooth muscle, cartilage, nor thyroid tissue could be identified. These histologic findings were consistent with the diagnosis of cutaneous bronchogenic cyst.
Cutaneous bronchogenic cysts are extremely rare congenital anomalies; only about 50 cases have been described in the literature. The first case of a cutaneous bronchogenic cyst was a lesion in the presternal region reported by Seybold and Clagett in 1945, although many cases of mediastinal bronchogenic cysts had already been reported by that time. In most cases, the lesions are noted either shortly after birth or in early childhood, and present as swelling or a draining sinuses. Such cysts are more prevalent in boys than in girls, by a ratio of almost 4:17. The cysts are usually observed near the suprasternal notch or the manubrium sterni; they can also be found in the neck, chin, base of the tongue, shoulder, and scapular regions4,8. Rarer locations include the chin, posterior neck, and abdominal wall. Clinically, most patients have an asymptomatic soft mass or draining sinus tract in the extra-thoracic area that is first noted in infancy or childhood. A fistulous tract may connect to the epidermis. A bronchogenic cyst is a benign congenital/developmental abnormality of the embryonic foregut. Abnormal development of the septum between the dorsal and ventral foregut buds may lead to the tracheoesophageal fistula, whereas when abnormal development occurs in the distal tracheobronchial tree, bronchogenic cysts can form.
Histologically, bronchogenic cysts are lined by ciliated, pseudostratified, columnar epithelium, with goblet cells. Smooth muscle, submucosal glands, and cartilage are present in 80%, 53%, and 7% of these cysts, respectively1.
Bronchogenic cysts should be included in the differential diagnosis of congenital cystic and nodular skin lesions on the upper chest and neck areas. Diagnosis is established on the basis of both histopathologic evaluation and cyst location. The differential diagnosis includes thyroglossal duct cysts, cutaneous ciliated cysts, and branchial cysts. Thyroglossal duct cysts present as midline cystic nodules on the anterior neck in children or young adults. They arise from remnants of the thyroglossal duct. Histologically, thyroglossal duct cysts may be lined with cuboidal, columnar, or stratified squamous epithelium, and may contain some ciliated columnar cells. The characteristic histologic feature is the presence of thyroid follicles, characterized by low cuboidal cells, surrounding a homogeneous pink material in the cyst wall. Cutaneous ciliated cysts are uncommon cysts that typically occur on the lower extremities of young women, although a few cases have been reported in men. Histologically, cutaneous ciliated cysts may be unilocular or multilocular. The cyst wall is composed of simple, cuboidal-to-columnar ciliated epithelium, that frequently has papillary projections into the cyst lumen. Branchial cleft cysts occur in the preauricular area, mandibular region, or along the anterior border of the sternocleidomastoid muscle. Branchial cleft cysts most commonly present in the second or third decade of life. Histologically, these cysts are lined by stratified squamous epithelium or by pseudostratified ciliated columnar epithelium, and they are surrounded by lymphoid tissue.
Although reports of cutaneous bronchogenic cysts are not rare, they have hardly ever been considered in the clinical differential diagnosis of the reported cases. Zvulunov et al.2 reviewed 54 cases, and summarized one of the most different histologic features between bronchogenic cysts and branchial cleft cysts, i.e. the presence of lymphoid tissue. All cases of the reported bronchogenic cysts did not have lymphoid tissues. But, the cases of branchial cleft cysts predominantly showed the lymphoid tissues (>90%). The findings in our patient, which included a congenital cyst in a presternal location with respiratory epithelium seen on microscopy, indicate the bronchogenic origin of the cyst. However, the absence of other tissue usually found in bronchogenic cysts (e.g. smooth muscle, submucosal glands, and cartilage), and the presence of multiple lymphoid follicles, precluded the clear-cut identification by histology. The presence of lymphoid follicles suggested that the lesion was of branchial origin. Not only is this a classic criterion for branchial anomalies, but the presence of lymphoid tissue has often been thought to exclude bronchogenic origin3-5. These findings are no longer believed to be mutually exclusive, because branchial anomalies without lymphoid tissue have been reported, as have bronchogenic lesions with minimal to moderate amounts of lymphoid follicles7,9. In our case, the cyst had drained for a long time and became secondarily infected. In addition, the inflammatory cells were present around the wall of the cyst. Also, in the cyst with extensive infiltration of inflammatory cells, small mucosal papillary or polypoid projections, filled with the inflammatory cells, jutted into the lumen. We suggest that these changes may cause the lymphoid follicles in the bronchogenic cysts.
Therefore, the strongest criterion for identification of this lesion is location. When pathology fails to provide distinction, the presternal (i.e. midline) presentation may be sufficient proof of the bronchogenic origin1,4,5. Not only is this location unusual in a branchial anomaly (which usually prefers the anterior border of the sternocleidomastoid muscle), but actually is favored in the bronchogenic derivative3. Although a defect in the convoluted fistular path of the fourth branchial anomaly may explain such a caudal and central presentation, the bronchogenic origin of the cyst is a more plausible explanation10. In addition, Shah et al.11 showed that a total of 86 cutaneous or subcutaneous bronchogenic cysts have been reported in the English literature. The most common location for subcutaneous cysts was the suprasternal notch (30%), followed by the anterior neck, the scapular, and presternal areas. 6 cases of cutaneous bronchogenic cyst have been reported in the Korean literature, and 4 of them occurred in the peristernal area (67%)12-17. In contrast, 8 cases of cutaneous branchial cleft cyst have been reported in the Korean literature, and all of them occurred either in the mandible or in the neck (Fig. 4).
Exclusion of this lesion from the differential diagnosis of masses or sinuses presenting in the suprasternal region may prevent complete excision of its components, which may extend deep into the site of origin. The possibility of extension into the mediastinum should be eliminated by evaluation of chest X-rays and, if necessary, by magnetic resonance or ultrasound examinations. These examinations may assist in planning the operative approach18.
Figures and Tables
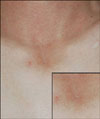 | Fig. 1A slightly erythematous nodule, with central pin-point sized opening on the suprasternal notch. |
 | Fig. 3(A) The excised specimen showed a cystic structure lined with ciliated pseudostratified columnar epithelium in the deep dermis and subcutis (H&E, ×100). (B) In the connective tissue surrounding the cystic cavity, several well-circumscribed basophilic lymphoid follicles were aggregated (H&E, ×200). (C) Goblet cells were scattered among the ciliated epithelial cells (H&E, ×400). (D) PAS-positive goblet cells were scattered among the ciliated epithelial cells (PAS, ×400). |
References
1. Fraga S, Helwig EB, Rosen SH. Bronchogenic cysts in the skin and subcutaneous tissue. Am J Clin Pathol. 1971. 56:230–238.

2. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998. 15:277–281.

3. Coleman WR, Homer RS, Kaplan RP. Branchial cleft heterotopia of the lower neck. J Cutan Pathol. 1989. 16:353–358.

4. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984. 11:367–371.

5. Søhoel P, Blom P, Mair IW. Subcutaneous bronchogenic anomalies. Ann Otol Rhinol Laryngol. 1980. 89:75–77.

6. Beyer LG, English JC 3rd, Halbach DP. Presternal bronchogenic sinus with predunculated lymphoid aggregate. Am J Dermatopathol. 2000. 22:79–82.
7. Pul N, Pul M. Bronchogenic cyst of the scapular area in an infant: case report and review of the literature. J Am Acad Dermatol. 1994. 31:120–122.

8. Tresser NJ, Dahms B, Berner JJ. Cutaneous bronchogenic cyst of the back: a case report and review of the literature. Pediatr Pathol. 1994. 14:207–212.

9. Bhaskar SN, Bernier JL. Histogenesis of branchial cysts; a report of 468 cases. Am J Pathol. 1959. 35:407–443.
11. Shah SK, Stayer SE, Hicks MJ, Brandt ML. Suprasternal bronchogenic cyst. J Pediatr Surg. 2008. 43:2115–2117.

12. Lee SN, Whang KK, Kim HI. Cutaneous bronchogenic cyst. Korean J Dermatol. 1986. 24:420–423.
13. Kang IK, Chung BS, Choi KC. A case of cutaneous bronchogenic cyst. Korean J Dermatol. 1991. 29:658–661.
14. Kang MJ, Shin NL, Cho SY, Hahm JH. A case of cutaneous bronchogenic cyst with lymphoid follicles. Korean J Dermatol. 1999. 37:1087–1090.
15. Lee SJ, Lim TG, Won DH, Kim YK, Choi GS. A case of cutaneous bronchogenic cyst. Korean J Dermatol. 2001. 39:928–929.
16. Park HS, Son HJ, Kang MJ. Cutaneous bronchogenic cyst over the sternum: a case report. Korean J Pathol. 2004. 38:333–336.
17. Park YK, Hur J, Chae SW, Sohn JH, Yoon DK. Cutaneous bronchogenic cyst of the anterior chest wall. J Korean Surg Soc. 2001. 60:678–680.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download