Abstract
Keratoacanthoma (KA) is a benign epidermal tumor, characterized by rapid and abundant growth, a tendency toward spontaneous regression and histopathologic similarity to squamous cell carcinoma (SCC). Because KA can be easily misdiagnosed as SCC, surgery is considered the treatment of choice. Recently, regression of KAs following application of 5% imiquimod cream (Aldara®) has been reported. We present 4 cases of KA treated with topical imiquimod, applied 3 to 4 times a week. Obvious improvement was observed after 4 to 6 weeks of application and the lesions were almost cleared leaving scars after 9 to 11 weeks. These results show that topical imiquimod can be an effective option for the conservative management of KA as previously reported. We also suggest that lesions treated with imiquimod cream should be considered for biopsy to judge histopathological remission after 5 to 8 weeks of application to shorten the duration of the treatment.
Keratoacanthoma (KA) is a benign epidermal tumor, characterized by rapid and abundant growth, a tendency toward spontaneous regression, and histopathologic similarity to squamous cell carcinoma (SCC). Since the first case of solitary KA was reported in 1889, there have been debates on whether KA is benign or not, because of its histological resemblance to SCC1. In typical cases, the policy of watchful waiting may be adopted, because KA usually regresses spontaneously. However, because of the frequent misdiagnosis of SCC as KA and the possibility of destruction of cosmetically significant organs, treatments have usually been recommended. Therapeutic options include complete excision, radiation therapy, intralesional injection of chemotherapeutic agents, oral retinoids and photodynamic therapy1. Recently, there are a few reports of successful treatment of KA by applying 5% imiquimod cream (imidazolquinoline, Aldara®). Here we present 4 cases of KA successfully treated with topical imiquimod.
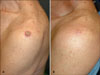
An 82-year-old Korean man presented with a well-demarcated round nodule on the left shoulder. The lesion appeared 1 month earlier. The physical examination revealed a well-circumscribed, 1.2 cm round nodule with a central ulcer on his left shoulder (Fig. 1A). He was healthy but a light smoker. After histopathological evaluation, the diagnosis of KA was confirmed. After 6 weeks of treatment with the imiquimod cream three times per week, the lesion significantly regressed. After 11 weeks, it was completely cleared (Fig. 1B). There were neither scars nor recurrence after 4 years follow-up.
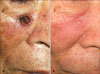
A 62-year-old Korean woman presented with a walnut-sized black-crusted crateriform tumor on her nose that had been present for a few months (Fig. 2A). The lesion was previously treated with carbon dioxide laser, but it recurred. She was systemically well and no enlarged lymph node was found. After histopathological study, the diagnosis of KA was confirmed. We treated her with applying imiquimod cream 3 times a week. After 6 weeks, the skin lesion remarkably decreased in size. After 10 weeks, the lesion was completely cleared leaving a scar (Fig. 2B). The application was maintained for another 2 weeks. She remained asymptomatic over a 10-month follow-up.
A 64-year-old man came to our clinic complaining of a rapidly growing firm round erythematous 1.2 cm sized nodule with a keratin plug on his right cheek (Fig. 3A), which had appeared 3 weeks ago. He had hypercholesterolemia and benign prostatic hypertrophy. We diagnosed it as KA clinically from the short history and the typical picture, and imiquimod cream was applied 3 times a week. Inflammatory reaction appeared after 2 weeks of application, but we encouraged him to continue the treatment. The tumor remarkably regressed after 5 weeks. After 9 weeks, the lesion was completely cleared leaving a scar. The treatment was maintained for another 4 weeks (Fig. 3B). No new lesions were seen at 1-year follow-up.
An 85-year-old man presented with a well-circumscribed firm dome-shaped ulcerative nodule of 1.5 cm diameter on his right cheek (Fig. 4A). The lesion first appeared 3 months earlier and was treated 2 times with intralesional methotrexate injection, but relapsed. Histopathological examination suggested KA, but a highly differentiated SCC could not be completely excluded. We treated him with the imiquimod cream application 3 to 4 times a week. Obvious improvement was observed after 4 weeks. After 10 weeks, the lesion was completely cleared leaving a scar. The treatment was continued for another 2 weeks (Fig. 4B) and no recurrence was observed over a period of 6 months.
Solitary KA, the most common subtype of KA, is a rapidly growing tumor that reaches 10 to 25 mm in diameter in 6 to 8 weeks2,3. It develops into a firm dome-shaped flesh-colored tumor with a central keratin-filled crater. After rapid proliferation, a mature KA undergoes regression in 4 to 6 weeks, leaving an atrophic and hypopigmented scar4,5. This process from proliferation to regression usually takes about 4 to 9 months, but there are some persistent cases which last for over 1 year1.
KA is regarded as a tumor which is derived from follicular infundibulum6. This explains its common involvement to the hair-bearing areas, like the face, neck, and hands1. However, keratin analyses of KA show the characteristics of both follicular differentiation and SCC7. In addition, KA usually demonstrated a histopathologic pattern often resembling that of a typical SCC, and there is no criterion to distinguish KA from SCC with sufficient sensitivity and specificity7. Furthermore, local destructions following rapid growth and metastases to other organs were observed in a few cases, although they had a tendency to spontaneously regress. In addition, treatment minimizes scarring which helps better cosmetic results. Therefore, treatment is recommended in most cases.
Complete surgical excision is the treatment of choice, but complete excision can be too destructive and cosmetically or functionally unacceptable for tumors on cosmetically important sites. There are many other treatment options of KA with various outcomes, such as cryotherapy, radiotherapy, intralesional injection of chemotherapeutic agent or interferon alpha, and topical 5-fluorouracil8 with a variable success rate.
These treatment options have some limitations. Surgical interventions (laser-, electro- and cryo-surgery) may also lead to substantial defects with functional or cosmetic morbidity, and may not allow the histopathologic confirmation of the clinical diagnosis. Radiotherapy is an effective treatment of KA9, but it is inappropriate for younger patients and is inconvenient because of the need for multiple visits to the hospital. Intralesional injection of chemotherapeutic agent has also proved therapeutically successful10. However, intralesional methotrexate therapy can have adverse events like pancytopenia, so a complete blood cell count should be considered to monitor for potential cytopenia. Also, intralesional 5-fluorouracil requires anesthesia for local pain control, with injections performed at consecutive week intervals10.
Recently, there are some reports of successful treatment with topical imiquimod (Table 1)11-17, a widely used topical immunomodulator in the group of toll-like receptor 7 and 8 agonist. Four to 11 weeks of application were required for the treatment, and sometimes adverse events which depended on the inflammation resulting from the immunological reaction, such as burning sensation, erythema and erosions occurred. In spite of these inconveniences, KA can be treated with topical imiquimod, because of lower invasiveness, non-inferiority in functional or cosmetic outcome and recent cases of successful treatment with topical imiquimod.
We analyzed 18 cases of KA treated with topical imiquimod (previously reported cases and ours). Data were statistically analyzed with a Mann-Whitney test using the SPSS version 17.0 statistical package (SPSS, Chicago, IL, USA). There were no statistically significant differences between previously reported cases and ours, except in the period of time to gain complete remission (p=0.005). The medians of the duration to complete remission were 6 weeks in 14 previously reported cases (range of 4 to 11 weeks), and 10 weeks in our 4 cases (range of 9 to 11 weeks).
Frequent application of imiquimod at the initial treatment was reported to induce a prompt regression of KA15. However, the analysis of previously reported cases showed no statistically significant difference in the duration to remission between cases applied once per day (median: 6.5 weeks; range of 5 to 8 weeks) and less than once per day (median: 6 weeks; range of 4 to 11 weeks; p=0.755). Similarly, the duration to complete remission was not related to age, size and the duration of KA.
The longer duration to complete remission in our cases may be caused by lack of histopathologic confirmation of remission, not by the frequency of application of initiation therapy. The duration required for clinical complete remission may be longer than that of histopathological remission, because the inflammation induced by imiquimod can make it difficult for clinicians to judge clinical cure. Mature KA undergoes regression in 6 weeks and topical imiquimod can promote the regression of KAs13. Furthermore, in previous cases of KA treated with imiquimod (Table 1), the average duration to obvious improvement was 5 weeks, and that to complete remission was 7.4 weeks. Therefore, after 5 to 8 week application, the lesions should be considered for biopsy to judge histopathological cure, if serial biopsies are not acceptable cosmetically.
In conclusion, topical imiquimod can be an effective option for the non-operative management of KA. For shortening the duration of the treatment, the histopathological confirmation of complete remission should be suggested. Further study is needed to investigate effective application frequency and duration of maintenance.
Figures and Tables
Fig. 1
(A) The lesion of case 1 on the left shoulder before therapy. (B) The lesion regressed completely after 11 weeks.
References
1. Karaa A, Khachemoune A. Keratoacanthoma: a tumor in search of a classification. Int J Dermatol. 2007. 46:671–678.

2. Beham A, Regauer S, Soyer HP, Beham-Schmid C. Keratoacanthoma: a clinically distinct variant of well differentiated squamous cell carcinoma. Adv Anat Pathol. 1998. 5:269–280.
3. Kwittken J. A histologic chronology of the clinical course of the keratocarcinoma (so-called keratoacanthoma). Mt Sinai J Med. 1975. 42:127–135.
5. Seifert A, Nasemann T. Keratoacanthoma and its clinical variants. Review of the literature and histopathologic analysis of 90 cases. Hautarzt. 1989. 40:189–202.
6. Yoshikawa K, Katagata Y, Kondo S. Relative amounts of keratin 17 are higher than those of keratin 16 in hair-follicle-derived tumors in comparison with nonfollicular epithelial skin tumors. J Invest Dermatol. 1995. 104:396–400.

8. Yuge S, Godoy DA, Melo MC, Sousa DS, Soares CT. Keratoacanthoma centrifugum marginatum: response to topical 5-fluorouracil. J Am Acad Dermatol. 2006. 54:5 Suppl. S218–S219.

9. Vergara A, Isarría MJ, Domínguez JD, Gamo R, Rodríguez Peralto JL, Guerra A. Multiple and relapsing keratoacanthomas developing at the edge of the skin grafts site after surgery and after radiotherapy. Dermatol Surg. 2007. 33:994–996.

10. Annest NM, VanBeek MJ, Arpey CJ, Whitaker DC. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007. 56:989–993.

11. Dendorfer M, Oppel T, Wollenberg A, Prinz JC. Topical treatment with imiquimod may induce regression of facial keratoacanthoma. Eur J Dermatol. 2003. 13:80–82.
12. Bhatia N. Imiquimod as a possible treatment for keratoacanthoma. J Drugs Dermatol. 2004. 3:71–74.
13. Di Lernia V, Ricci C, Albertini G. Spontaneous regression of keratoacanthoma can be promoted by topical treatment with imiquimod cream. J Eur Acad Dermatol Venereol. 2004. 18:626–629.

14. Kim YJ, Hwang ES, Son SW, Kim IH. Topical treatment with 5% imiquimod for solitary keratoacanthoma. Korean J Dermatol. 2004. 42:1321–1324.
15. Ko NY, Park JH, Son SW, Kim IH. Treatment of keratoacanthoma with 5% imiquimod cream. Ann Dermatol. 2006. 18:14–17.

16. Calista D, Morri M. Topical imiquimod for the treatment of keratoacanthomas. Eur J Dermatol. 2008. 18:590–591.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download