Abstract
Background
Although acne is a common follicular inflammatory dermatosis, studies of the relationship between Malassezia yeasts and acne have rarely been conducted.
Objective
We sought to identify Malassezia yeasts from acne patients and establish a relationship between specific types of species of Malassezia and acne.
Methods
Sixty acne patients were enrolled. Each strain obtained was identified as one of eleven species by 26S rDNA PCR-RFLP. We then compared these data with those of age- and sex-matched healthy subjects.
Results
Growth of Malassezia was evident in fewer patients with acne (50%) in comparison to controls (70.6%). M. restricta was dominant in patients with acne (23.9%), whereas M. globosa was most common (26.7%) in healthy controls. In the patients group, the rate was the highest (71.7%) in the twenties and, in terms of body site, the rate was the highest (60%) in the chest. In the control group, the rate was the highest (75.0%) in the thirties and in the forehead (85.0%).
Acne is a chronic, self-limited inflammatory disorder of the pilosebaceous unit that peaks during puberty, the period in which androgen production is suddenly increased. It commonly affects the face, neck, and chest. It is known to be caused by increased sebum production, due to the influence of androgen, hyperkeratosis of the entry site of the follicle, and induction of inflammatory mediators owing to growth of P. acnes1.
Types of resident flora vary widely, depending on location. The infra-infundibulum harbors P. acnes, while the acroinfundibulum is populated by Malassezia yeasts, and the skin surface around the follicle by aerobic Staphylococcus epidermidis2,3. Among these, Malassezia yeasts are a lipophilic fungus classified as normal flora, and are isolated in 75~80% of healthy subjects4,5. However, as morphologic, immunologic, physiologic, and molecular biological studies progressed, the need for new classification of the species was brought forward. Thus, in 1996, Guého et al. re-classified the yeasts into seven species (M. furfur, M. obtusa, M. globosa, M. slooffiae, M. sympodialis, M. pachydermatis, and M. restricta) based on morphologic, microscopic, physiologic, and molecular biological characteristics6-10. Also, recently, in Japan, four new species, M. dermatis, M. japonica, M. nana, and M. yamatoensis, and in Europe, two additional species, M. caprae, and M. equina, have been identified; therefore, the yeasts are now classified into thirteen species11-15.
They are known to be linked to pityriasis versicolor, seborrheic dermatitis, Malassezia folliculitis, and, most recently, atopic dermatitis and psoriasis16-18. Although the presence of Malassezia yeasts in both the comedon and follicle of acne patients has been confirmed19, its exact role in the pathogenesis of acne is not clearly defined.
Thus, the authors were motivated to engage in a study of the distribution of Malassezia yeasts in patients with acne, using molecular biological techniques. We also sought to determine whether there is any difference in the distribution of Malassezia yeasts between patient and control groups, through comparison with sex- and age-matched controls from the previous study.
The patients group consisted of 60 acne patients aged 11 to 40 who visited Konkuk University Hospital, Department of dermatology, between July 2005 and December 2009. The patient group was classified into teens, twenties, and thirties subgroups, with each subgroup consisting of 20 patients. All patients were age- and sex-matched with sixty healthy controls. Subjects with dermatologic problems other than acne were excluded from the study. Those who were on systemic adrenocorticoid therapy, phototherapy, or antifungal agents within the past two months, as well as those who had been treated with topical antifungal agents within the past month, and/or with topical corticosteroid within one week, were also excluded from the study. All subjects were instructed to avoid the use of moisturizers and facial cleansing on the day of examination, and informed consent was obtained from each individual after a thorough explanation of possible physical and psychological adverse outcomes that could arise during the course of the study. This study was conducted in strict abidance with the Helsinki principles.
The forehead, cheek, and chest, which are common locations of acne, were chosen for sampling, and, whenever possible, samples were taken from actual acne lesions.
The LNA (Leeming-Notman agar) medium was prepared by mixing glycerol monoesterate (BDH, Poole, UK) 0.5 g, bacteriological peptone (Oxoid, Hampshire, UK) 20 g, glucose (Oxoid, Hampshire, UK) 5 g, yeast extract (Oxoid, Hampshire, UK) 0.1 g, ox bile (Merck, Darmstadt, Germany) 4 g, agar No.1 (Oxoid, Hampshire, UK) 12 g, Tween 60 (Yakuri, Osaka, Japan), 0.5 ml, and glycerol (Tedia, Fairfield, USA) 1 ml with one liter of distilled water and sterilizing it for twenty minutes at 121℃. Following the sterilization process, cycloheximide (Sigma, St Louis, MO, USA) 200 mg, and chloramphenicol (Sigma, St Louis, MO, USA) 50 mg were added, followed by 5 ml of non-skim milk treated at a super-high temperature (Konkuk Dairy, Seoul, Korea). After thorough mixing, the solution was spread evenly on a Petri dish and kept refrigerated until use. The washing solution was prepared by dissolving NaH2PO42H2O 1.17 g in 100 ml of distilled water, 85 ml of which was taken and mixed with Na2HPO4 10.6 g and 1,000 ml of distilled water. The pH was adjusted to 7.9, and 1 ml of Triton X100 was added to the solution before sterilizing at 121℃ for twenty minutes and refrigerating.
Specimens were harvested from the forehead, cheek, and chest, and were sampled by a scrub-wash technique, based on the method suggested by Williamson and Kligman20. A stainless tube, with an interior area of 4.909 cm2, was set on the selected part of the skin, i.e. forehead, cheek, and chest, and then 1 ml of detergent16 (0.01% NaH2PO42H2O, 1.01% Na2HPO4, 0.1% Triton X-100 [pH 7.9]) was added to the tube. After rubbing the skin with a glass rod for one minute, the sample was removed using a pipette and stored in a different container. One ml of the detergent was then added to the stainless tube, and the specimen was sampled repetitively and added to the first sample. One hundred microliters of the sampled specimen was then mixed with 900µl of the detergent with a 50% concentration, and 100µl was taken from the mixture, evenly applied on the Leeming-Notman medium, and cultured at 34℃ for 14 days21.
For DNA extraction and PCR analysis of skin isolates, we adopted the colony PCR analysis10, which was developed for extraction of DNA directly from a colony of a PCR tube and for amplification of 26S rDNA at the same time, instead of direct genomic DNA extraction methods. A colony of Malassezia yeast was removed and transferred to a PCR tube, then warmed 3 times a day for 1 minute, in a double boiler, using a microwave; the tube was then immersed in ice water. The PCR reaction mixture (0.25 mM deoxynucleoside triphosphate, 10X PCR buffer, 5X Q buffer, 0.5µM primers, 1.25 U Hot StarTaq polymerase, 20 mM MgSO4) was added and vortex mixing was performed. PCR, using the Mastercycler 5333 (Eppendorf, Hamburg, Germany), was then performed immediately22,23. For amplification of 26S rDNA, a primer capable of amplifying all 11 standard strains at once was chosen. The sequence was as follows: forward, 5'-TAACAAGGATTCCCCTAGTA-3' and reverse, 5'-ATTACGCCAGCATCCTAAG-3'. Conditions in the early stage of the reaction were at 95℃ for 14 minutes for pre-denaturation, at 94℃ for 45 seconds for denaturation, at 55℃ for 45 seconds for annealing, at 72℃ for 1 minute for extension of 40 cycles, and at 72℃ for 7 minutes for the last extension. Amplified DNA was visualized by electrophoresis on a 1.5% (w/v) agarose gel with Ethidium bromide (0.5µg/ml), using 1X TAE migrating buffer (pH 8.0, 40 mM Tri-acetate 1 mM EDTA).
After checking the amplified 26S rDNA, the product of PCR was purified using the Accu-Prep PCR purification kit (Bioneer, Daejeon, Korea). For 26S rDNA RFLP analysis of Malassezia yeasts, two restriction enzymes were used: Hha I (Takara Biomedicals, Otsu, Japan) and BtsF51 (SibEnzyme, Novosibirsk, Russia). The restriction enzyme reaction was conditioned with 10X PCR buffer, 10U restriction enzyme, and reaction mixture, including 7.5µl of the PCR product to become 20µl. After three hours of reaction at 37℃, electrophoresis was performed in TAE buffer with 3.5% (w/v) NuSieve GTG agarose gel (FMC, Rockland, ME, USA) by 100 volts. It was then stained with ethidium bromide, and the size and number of DNA fragments were measured by a UV transilluminator for analysis of RFLP patterns23(Fig. 1).
Malassezia yeasts were cultured in 90 out of 180 samples collected from the forehead, cheek, and chest of 60 acne patients, yielding a 50% isolation rate, and a total of four species of Malassezia yeasts (i.e. M. restricta, M. globosa, M. sympodialis, and M. furfur) were isolated. On the other hand, a total of five species (M. globosa, M. restricta, M. sympodialis, M. dermatis, and M. furfur) were recovered in the healthy control group. In terms of age, for the patients group, the culture rate was the highest in the twenties group (i.e. age 21 to 30), with 71.7% (43 of 60), followed by the teens group (24 of 60, 40%), and the thirties group (23 of 60, 38.3%). In the control group, the rate was the highest in the thirties (age 31 to 40), with 75% (45 of 60) (Table 1). In terms of body sites, for the patients group, the rate was the highest in the chest, with 60% (36 of 60), followed by 50% (30 of 60) in the forehead, and 40% (24 of 60) in the cheek. In the controls, the rate was the highest in the forehead, with 85% (51 of 60) (Table 2).
Upon stratification of the isolated species of Malassezia yeasts according to age in the patients group, M. restricta was the most frequently isolated species in the twenties, with 46.7%, and M. globosa was most commonly identified in the thirties, with 13.3%; in the teens, M. restricta, M. globosa, and M. sympodialis were recovered in equal proportions (13.3%). In contrast, for the control group, M. globosa was the most predominant species in the teens (28.3%) and the twenties (33.3%) subgroups, while M. sympodialis was most commonly found in the thirties (25.0%) (Fig. 2). A statistically significant difference was observed between the patient and control groups in the twenties and thirties (Table 3).
Upon stratification of the species of Malassezia yeasts according to body sites in the acne patients, M. restricta was most commonly isolated, with 43 cases (23.9%), which was higher than the rate in the control group, with 38 cases (21.1%). In the forehead, M. restricta was found in 20%, M. globosa in 20%, and M. sympodialis in 8.3%. In the cheek, M. restricta was isolated in 16.7%, M. globosa in 15%, and M. sympodialis in 6.7%. In the chest, M. restricta was recovered in 35%, M. sympodialis in 13.3%, and M. globosa in 11.7%. In the control group, M. globosa was most commonly isolated overall (48 of 180, 26.7%) (Fig. 3). Also, a statistically significant difference between the two groups was observed in two body sites, but not in the cheek (Table 4).
Acne is a chronic, self-limited inflammatory disorder of the pilosebaceous unit that peaks during puberty, when androgen production is suddenly increased. It commonly affects the face, neck, and chest. It is known to be caused by increased sebum production through the influence of androgen, hyperkeratosis of the entry site of the follicle, and induction of inflammatory mediators due to growth of P. acnes1. Clinically, it manifests as various non-inflammatory lesions, such as comedon, papule, pustule, and nodule24. Although far from life-threatening, its potential consequences should not be underestimated, for it causes embarrassment, depression, low self-esteem, social withdrawal, and poor quality of life.
The organisms found in the acne lesions, alongside P. acnes, include Malassezia. Given the fact that Malassezia yeasts are capable of forming comedones, it is likely that Malasszia folliculitis might actually co-exist with deep acnes of the face, and many cases labeled "deep-seated acnes" might in fact have been Malassezia-associated lesions19,25. Also, medications for treatment of acne decrease the number of P. acnes, while the relative number of Malassezia yeasts increases; thus, treatment might seemingly result in no improvement26. In fact, according to one report, antifungal treatment is effective in cases of deep acnes with known presence of Malassezia yeasts2,27.
Overall, when compared to normal controls, the isolation rate of Malassezia yeasts was conspicuously low in the acne patients group. This observation may be attributable to the fact that overgrowth of P. acnes may have brought about a relative decrease in the number of Malassezia yeasts. In this study, the age of the patients group was limited to the teens, twenties, and thirties. This is because the majority of cases affect ages 12 to 25. The pre-study hypothesis was that the isolation rate of Malassezia yeasts would be particularly lower in the teens and twenties, compared to that of the thirties; however, our study revealed that the isolation rate was rather higher in groups of patients in their teens and twenties, a finding that may be attributable to variation in the severity of skin lesions in the patients group.
In general, the most frequent site of acne is the face, followed by the back, chest, and neck. The cheek is the location where acne lesions are most severe, both in adults and adolescents. Also, in adults, the frequency of acne is known to be highest in the chin and around the mouth28. This led to speculation that the detection rate would be lower in the cheek, where the number of lesions is relatively higher, compared to the chest. In our study, the rate of isolation was lower in the cheek and the forehead, where the number of acne lesions is relatively high, compared to the chest.
According to the report by Kang and Kim19, who conducted a direct smear test and culture for identification of the causative organisms on the facial comedones of patients diagnosed with acne vulgaris, the most frequently isolated organism in acne is M. restricta (28%), followed by M. globosa (13%). The results of the present study were very similar, with M. restricta being recovered in 23.9% and M. globosa in 15.6%. In particular, in the case of M. restricta, the rate was conspicuously higher in the twenties group, and on the chest in the patients group, and the difference was statistically significant. This cautiously brings up the possibility of M. restricta having a relationship with acne lesions of the chest. However, to this point, there has not been a sufficient number of studies on the relationship between development of acne and species of Malassezia yeast, which would support the results of this study. Also, this might be due to technical errors while sampling specimens, e.g. isolation of the strains from lesions that may be clinically confused with acnes (e.g., Malassezia folliculitis, miliaria pustulosa etc.). Malassezia folliculitis might be clinically confused with acne, for the main clinical manifestations are papules and pustules, with pruritus on the chest and back2. Malassezia folliculitis might coexist with acne lesions because the areas are equally populated by Malassezia yeasts, although the facial areas, such as the cheek, forehead, and chin, are the main locations of acne vulgaris lesions induced by P. acnes; furthermore, it may also be related to the report by Jacinto-Jamora et al.29 that lesions in the forehead and the chin are in fact Malassezia-associated.
Given the outcome of the study, we have found a few clues. However, to this point, there has been no report clearly stating the relationship between acne and Malassezia yeasts, nor was this study successful in identifying the relationship. Therefore, further studies comparing inflammatory from non-inflammatory lesions in the distribution of Malassezia yeasts, and using large-scale quantitative analysis, will hopefully guide us to elucidation of the relationship between acne and Malassezia yeasts.
Figures and Tables
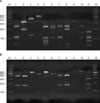 | Fig. 1PCR-RFLP patterns of 26S rDNA PCR digested with Hha I (A), and BstF51 (B) of 11 Malassezia standard strains. Lanes: M, molecular marker; 1. M. furfur (KCTC 7743); 2. M. sympodialis (KCTC 7985); 3. M. globosa (CBS 7966); 4. M. restricta (KCTC 7848); 5. M. slooffiae (KCTC 17431); 6. M. pachydermatis (KCTC 17008); 7. M. japonica (CBS 9432); 8. M. nana (JCM 12085); 9. M. dermatis (JCM 11348); 10. M. obtusa (KCTC 7847); 11. M. yamatoensis (CBS 9725). |
 | Fig. 2Identified Malassezia species from the acne group, compared by age with those from the healthy control group. |
 | Fig. 3Identified Malassezia species from the acne group compared by body site with those from the healthy control group. |
References
1. Zaenglein AL, Graber EM, Thiboutot DM, Strauss JS. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Acne vulgaris and acneiform eruptions. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;690–703.
2. Youn NH, Cha SH, Park SD. Malassezia yeasts in acne vulgaris. Korean J Dermatol. 2002. 40:1453–1460.
3. Leeming JP, Holland KT, Cuncliffe WJ. The microbial colonization of inflamed acne vulgaris lesions. Br J Dermatol. 1988. 118:203–208.

4. Janik MP, Heffernan MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Yeast infections: Candidiasis, Pityriasis (Tinea) versicolor. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1822–1830.
5. Ahn KJ. Taxonomy of the genus Malassezia. Korean J Med Mycol. 1998. 3:81–88.
6. Guillot J, Guého E. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie Van Leeuwenhoek. 1995. 67:297–314.
7. Guillot J, Gueho E, Lesourd M, Midgley G, Chevrier G, Dupont B. Identification of Malassezia species: a practical approach. J Mycol Med. 1996. 6:103–110.
8. Yamada Y, Makimura K, Ueda K, Nishiyama Y, Uchida K, Yamaguchi H, et al. DNA base alignment and taxonomic study of genus Malassezia based upon partial sequences of mitochondrial large subunit ribosomal RNA gene. Microbiol Immunol. 2003. 47:475–478.

9. Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek. 1996. 69:337–355.

10. Kim SM, Lim SH, Jung BR, Lee YW, Choe YB, Ahn KJ. The application of colony PCR in the molecular biological analysis of Malassezia yeasts. Korean J Med Mycol. 2007. 12:180–188.
11. Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002. 40:1363–1367.

12. Hirai A, Kano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, et al. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol. 2004. 54:623–627.

13. Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003. 41:4695–4699.

14. Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, et al. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Immunol. 2004. 48:579–583.

15. Cabañes FJ, Theelen B, Castellá G, Boekhout T. Two new lipid-dependent Malassezia species from domestic animals. FEMS Yeast Res. 2007. 7:1064–1076.
16. Ljubojević S, Skerlev M, Lipozencić J, Basta-Juzbasić A. The role of Malassezia furfur in dermatology. Clin Dermatol. 2002. 20:179–182.
17. Kanda N, Tani K, Enomoto U, Nakai K, Watanabe S. The skin fungus-induced Th1- and Th2-related cytokine, chemokine and prostaglandin E2 production in peripheral blood mononuclear cells from patients with atopic dermatitis and psoriasis vulgaris. Clin Exp Allergy. 2002. 32:1243–1250.

18. Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL Jr. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004. 51:785–798.
19. Kang SH, Kim HU. The isolation of Malassezia yeasts in the comedones of acne vulgaris. Korean J Med Mycol. 1999. 4:33–39.
20. Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965. 45:498–503.

21. Leeming JP, Notman FH, Holland KT. The distribution and ecology of Malassezia furfur and cutaneous bacteria on human skin. J Appl Bacteriol. 1989. 67:47–52.

22. Yamada Y, Makimura K, Merhendi H, Ueda K, Nishiyama Y, Yamaguchi H, et al. Comparison of different methods for extraction of mitochondrial DNA from human pathogenic yeasts. Jpn J Infect Dis. 2002. 55:122–125.
23. Lee YW, Lim SH, Ahn KJ. The application of 26S rDNA PCR-RFLP in the identification and classification of Malassezia yeast. Korean J Med Mycol. 2006. 11:141–153.
25. Seo YJ, Piao YJ, Suhr KB, Lee JH, Park JK. A comparative study on clinical and therapeutic features between Malassezia folliculitis and steroid acne. Korean J Med Mycol. 2001. 6:160–166.
26. Ayers K, Sweeney SM, Wiss K. Pityrosporum folliculitis: diagnosis and management in 6 female adolescents with acne vulgaris. Arch Pediatr Adolesc Med. 2005. 159:64–67.
27. Yu HJ, Kim YS, Yang HY, Kim JH, Lee SK, Son SJ. The incidences of Malassezia in steroid acne and other acneiform eruptions. Korean J Med Mycol. 1998. 3:24–32.
28. Kang MJ, Hahm JH. Comparative study of acne on clinical features and patient understandings in adolescence and post-adolescence. Korean J Dermatol. 2000. 38:589–599.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download