Abstract
Background
S100A12 is a member of the S100 family of calcium-binding proteins and is secreted either in inflamed tissues or in the bloodstream by activated neutrophils. Expression of S100A12 has been reported in various diseases, especially non-infectious inflammatory diseases, such as Kawasaki disease, giant cell arteritis and inflammatory bowel disease.
Objective
This study was conducted to determine both the tissue expression and the serum levels of S100A12 in Behçet's disease (BD) patients and the correlation of the S100A12 serum level with disease activity of BD.
Methods
We included in this study ten BD patients who fulfilled the criteria for diagnosis, according to the International Study Group for BD. The activity of BD was calculated using the BD Current Activity Form. The serum concentrations of both S100A12 and interleukin-8 were measured by the enzyme-linked immunosorbent assay, before and after treatment. Immunohistochemical studies were also performed to detect S100A12 expression in the skin.
Results
The serum S100A12 level was significantly increased in the active BD period (p<0.001), in the inactive BD period (p=0.041) and in patients with active Kawasaki disease (p=0.028), compared with the serum level in the healthy controls. The serum S100A12 level decreased significantly from baseline, compared to post-treatment (p=0.017). The activity score of BD was significantly correlated with the serum level of S100A12 (Spearman's coefficient=0.464, p=0.039). Immunohistochemical studies showed that S100A12 was strongly expressed in the erythema nodosum-like skin lesions of patients.
Behçet's disease (BD), also known as Adamantiades-Behçet's disease, is a recurrent, multisystemic inflammatory disease characterized by recurrent oral aphthous and genital ulcers, ocular lesions, and skin lesions, and is occasionally accompanied by articular, urogenital, vascular, gastrointestinal and neurologic system involvement1-3. Although genetic factors, infectious agents and immunological mechanisms have all been implicated and studied, the etiology of BD still remains to be elucidated4. The role of hyperactive neutrophils is important in the pathogenesis of BD; histopathologic findings of show that the erythema nodosum-like lesions of BD are mainly composed of neutrophilic vascular inflammation, with accompanying changes in the subcutis5-7. Jorizzo et al.7 suggested that, during the evolution of these lesions, the lymphocyte-predominating reaction might follow a neutrophilic vascular reaction. Also, increased chemotaxis, phagocytosis, superoxide and lysosomal enzyme production, as well as enhanced expressions of both CD11a and CD18 on the cell surfaces, have been reported in the neutrophils of BD patients8-10. These observations suggest that, in BD, the neutrophils are overactive and can eventually lead to tissue injury11,12.
S100A12, a newly identified extracellular receptor for advanced glycation end products-binding protein (EN-RAGE), is a member of the S100 family of calcium-binding proteins13. S100A12 is secreted either in inflamed tissues or in the bloodstream by activated neutrophils, and interacts with the multi-ligand RAGE, as a receptor transducing proinflammatory signals, in the endothelial cells and cells of the immune system. S100A12 has been reported to have potent chemotactic activity, comparable with other chemotactic agents14,15. Binding of S100A12 to the extracellular domain of membrane RAGE activates an intracellular signal cascade, including the nuclear factor (NF)-κB, and induces secretion of cytokines, such as tumor necrosis factor-α (TNF-α)13,15-17. Thereby, S100A12 mediates the proinflammatory effects on lymphocytes, endothelial cells, neutrophils and mononuclear phagocytes13,18. Expression of S100A12 has been reported in various disorders, especially non-infectious inflammatory conditions, including Kawasaki disease (KD), inflammatory bowel disease (IBD), and giant cell arteritis19-22. Neutrophil infiltration and activation has been observed in the coronary artery lesions of KD, inflamed mucosa of IBD, and vasa vasorum within the adventitial layer of giant cell arteritis18-22. S100A12 is overexpressed at local sites of inflammation, and a high concentration of S100A12 can be found, during an active inflammatory episode13,18, in the serum, plasma, synovial fluid and stool. According to previous studies, in individual patients23, the S100A12 level is a sensitive marker for monitoring both the disease activity and the response to treatment. Both the anti-S100A12 antibodies and soluble RAGE have been reported to suppress inflammatory reactions in the murine model of bovine serum albumin-induced hypersensitivity and chronic bowel inflammation24,25. However, to the best of our knowledge, the relationship between S100A12 and BD has not yet been described. The aim of our study was to determine both the tissue expression and the serum levels of S100A12 in BD patients. We also investigated, in BD patients, the changes in the serum levels of S100A12 according to disease activity.
We conducted this prospective pilot study following approval by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. A total of ten BD patients (mean age 38.6±9.2 years; two male and eight female), who fulfilled the criteria for diagnosis from the International Study Group for BD, were enrolled in this study26. During study participation, all patients had been in the active period of the disease on their initial presentation, as demonstrated by fulfilling at least two of the following criteria, within four weeks of the study period: oral ulcer, genital ulcer, skin lesions, ocular lesions, arthritis, urogenital, vascular, gastrointestinal and neurologic system involvements. Patients were defined as being in the inactive period if they had none or one of the above criteria within four weeks. After the treatment, all patients were converted from the active to the inactive stage of BD. The activity of BD was calculated using the BD Current Activity Form 2006 (BDCAF; www.behcet.ws/pdf/BehçetsDiseaseActivityForm.pdf) and summarized in Table 1. The mean activity scores were separately evaluated in the baseline active period and in the inactive period, eight weeks after treatment. In the descending order of frequency, the following systemic treatments were delivered to the BD patients: colchicine (n=10), prednisolone (n=9), nonsteroidal anti-inflammatory drugs (n=7), azathioprine (n=2), minocycline (n=1) and sulfasalazine (n=1).
The serum samples were separately collected from each patient, during the active and inactive periods of BD. In addition, the sera from ten patients with active KD (mean age 2.5±1.2 years; six male and four female) were obtained, as the disease control group. The diagnosis of KD was made by a pediatrician based on the fulfillment of at least five of the six diagnostic criteria for KD, as previously presented27. The sera of ten age- and sex-matched healthy individuals (mean age 37.2±8.1 years; two male and eight female) were also collected, as the normal control group. In five of ten BD participants with active BD, two to four days after the appearance of the skin lesions, biopsy specimens were taken for immunohistochemical studies from the erythema nodosum-like skin lesions in the shin. The skin lesions were about 3~6 cm sized erythematous tender nodules. As a control group, buttock skin samples were obtained from five age- and sex-matched healthy volunteers, without any specific skin disease. Paraffin-embedded sections of the skin biopsies diagnosed as erythema nodosum (EN) were used, as previously described. Informed consent was obtained from all participants in this study.
Serum S100A12 and IL-8 levels were evaluated before and after treatment. For determination of the serum S100A12 and IL-8 levels, peripheral venous blood samples were drawn into vacutainer SST tubes (Becton Dickinson, Mountain View, CA, USA) and allowed to clot at room temperature (20℃ to 24℃) for 30 minutes. The tubes were centrifuged at 1,000× g for ten minutes. The sera were then aliquoted and stored at -70℃, until used for testing. The serum S100A12 and IL-8 levels were determined (in duplicate) using commercial S100A12 enzyme-linked immunosorbent assay (ELISA) kits (CycLex Co. Ltd., Nagano, Japan) and IL-8 ELISA kits (R&D Systems, Minneapolis, MN, USA). These procedures were performed according to the manufacturers' instructions.
Paraffin-embedded sections of skin biopsies were used to detect S100A12 expression. Specimens were immediately fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in paraffin. Commercially available purified mouse monoclonal anti-human S100A12 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used in the immunohistochemical staining. The detailed procedures are, as follows. Prior to staining, tissue slides were de-paraffinized with xylene and re-hydrated in a graded series of ethanol, to remove the embedding media. Then tissue sections were pre-treated by boiling in 10 mM sodium citrate buffer (pH 6.0) in a microwave (95℃ for two minutes), and washed and cooled in a phosphate-buffered saline (PBS) bath for 20 minutes. After washing, non-specific binding was blocked with 5% normal goat serum for 20 minutes. Then, tissue sections were incubated in a humidified chamber, with anti-human S100A12 antibodies for two hours at 4℃. After incubation with the primary antibody, sections were rinsed three times with PBS containing 0.05% Tween-20 for two minutes, and treated with biotin-conjugated secondary antibody for 30 minutes. After incubation with the secondary antibody, sections were rinsed three times with PBS containing 0.05% Tween-20 for two minutes, incubated with alkaline phosphate conjugate for ten minutes, and rinsed in the same way. Then we applied HRP-streptavidin reagent to each section, incubated them for 30 minutes, rinsed them with distilled water containing 0.05% Tween-20, and stained them with hematoxylin for counter-staining. Finally, the specimens were dehydrated with gradient alcohol and xylene and mounted with permanent mounting medium.
The Mann-Whitney U test and Wilcoxon signed-rank test were performed to determine significant differences between the distinct categories. Correlations were analyzed using the Spearman's correlation coefficient. p-values of less than 0.05 were considered statistically significant. Statistical analyses were performed using the Statistical Product and Service Solutions program version 15 (SPSS Inc, Chicago, IL) for Windows.
The serum level of S100A12 was significantly increased in the active BD period (median 1,134 ng/ml, range 373~1,211 ng/ml, p<0.001), in the inactive BD period (median 299 ng/ml, range 21~682 ng/ml, p=0.041), and in patients with active KD (median 356 ng/ml, range 54~1,677 ng/ml, p=0.028), compared to the serum level of the healthy controls (median 68 ng/ml, range 18~442 ng/ml) (Fig. 1). In the literature, mean serum S100A12 levels of healthy controls ranged from 10 ng/ml to 75 ng/ml13.
In BD patients, the median serum S100A12 level significantly decreased from 1,134 ng/ml (range 373~1,211 ng/ml) at baseline to 299 ng/ml (range 21~682 ng/ml) after treatment in the inactive BD disease state (p=0.017) (Table 1). However, one patient showed an increase in S100A12 even after treatment (Fig. 2). The mean activity scores, which were separately evaluated in the active period as baseline and, in the inactive period, eight weeks after treatment, were 3.7±1.3 (range 2~6) and 0.5±0.4 (range 0~1), respectively. The activity score was significantly correlated with the serum S100A12 level (Spearman's coefficient=0.464, p=0.039) (Fig. 3).
Serum IL-8 was significantly increased in the active BD period (median 932 pg/ml, range 176~2,207 pg/ml, p<0.001), but not in the inactive BD period (median 48 pg/ml, range 11~3,322 pg/ml, p=0.290), nor in KD patients (median 13 pg/ml, range 9~300 pg/ml, p=0.436), compared to the serum levels of healthy controls (median 15 pg/ml, range 10~208 pg/ml) (Fig. 4). In BD patients, after the treatment, the median serum IL-8 level decreased from baseline to 48 pg/ml (range 11~1,132 pg/ml) at the inactive period, but the decrease was not statistically significant (p=0.285). Two patients even showed an increase in the serum IL-8 level after treatment (Fig. 5). The activity score was not significantly correlated with the serum IL-8 level (Spearman's coefficient=0.285, p=0.110). There was no correlation between changes in serum IL-8 or S100A12 levels and systemic treatment modalities.
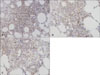
Immunohistochemical analyses of biopsy specimens from five BD patients demonstrated strong reactivity with anti-S100A12 antibody in the cytoplasms of lymphohistocytes, neutrophils, and endothelial cells, which all stained brown, whereas the cell nuclei were counterstained blue (Fig. 6). S100A12 was also distributed extracellularly, surrounding the anti-S100A12 antibody-reactive cells, which suggests that it is a secreted protein. In all biopsies examined, the extent of reactivity differed between cases, but the staining pattern was similar. Although, some lymphohistiocytes expressed S100A12 in EN specimens, the expression level was weaker than in the erythema nodosum-like skin lesions of BD patients. Only sparse S100A12 expression was observed in biopsy specimens obtained from healthy control subjects.
Neutrophil infiltration is an important characteristic of the active phase of BD. However, the mechanism underlying the hyperactivity of neutrophils in BD has not been fully elucidated. According to previous reports, Th1-type cytokines and chemokines, including IL-8, IL-17, TNF-α and interferon (IFN)-γ, have been suggested as a cause of neutrophilic hyperactivity28,29. Especially, elevated serum IL-8 levels have been demonstrated in BD patients30-32 during the active phase of the disease.
Activated granulocytes secrete S100A12, which is also a strong chemotactic factor for other leukocytes13,17-19,21. In the present study, analysis of S100A12 in both erythema nodosum-like skin lesions and sera indicated that S100A12 is expressed and secreted at local sites of inflammation. Infiltration of S100A12-positive polymorphonuclear cells is supposed to be an early step in the inflammation process, prior to invasion of mononuclear cells. Ligation of RAGE by S100A12 activates NF-κB and proinflammatory cytokines, including TNF-α, IL-2 and IL-613,18,33. In BD patients13,33, NF-κB activation may protect T cells against apoptosis, via expression of anti-apoptotic genes. It has been demonstrated that TNF-α, which is observed in high amounts in both skin lesions and sera of BD patients, stimulates S100A12 secretion in peripheral neutrophils, and also induces NF-κB activation29,34-37. Therefore, TNF-α and S100A12 are suggested to constitute a positive inflammatory feedback loop18. S100A12 has been shown to enhance the expressions of adhesion molecules on endothelial cells, including ICAM-1 and VCAM-117,18. In BD patients, some studies have reported that the expressions of ICAM-1 and VCAM-1 on the endothelial cells are increased, which is associated with enhanced endothelial adhesion properties of both mononuclear cells and neutrophils38,39.
The role of IL-8 in BD has been demonstrated in several investigations. IL-8 is a potent chemoattractant and activator of neutrophils26. Some studies in BD patients31-33 have shown increased serum levels of IL-8 that were correlated with disease activity. However, other contradictory results have indicated that IL-8 levels were not related to disease activity in BD patients40,41. In the present study, serum IL-8 was significantly increased in active BD, but not in the inactive BD period, compared with controls. Although the serum IL-8 levels decreased after systemic treatment, the change was not statistically significant, and serum IL-8 concentrations were not correlated with the activity score. Based on this finding, we concluded that, in our study population, S100A12 was a better indicator of BD activity than IL-8.
There have been few reports on the role of IL-8 in KD, although neutrophils may be early effector cells for vascular endothelial damage in the acute phase of KD. Lin et al.42 reported that the elevated serum IL-8 level during the first week of illness may be associated with a higher risk of coronary aneurysm formation. However, Suzuki et al.43 found no significant differences in the serum IL-8 levels, before and after intravenous immunoglobulin treatment. Moreover, Asano and Ogawa44 revealed that the plasma level of IL-8 was elevated in the acute phase of KD, compared with that of healthy controls, though the difference was not statistically significant. In our study, a significant increase in the serum S100A12 level was observed in both BD and KD patients, and the serum IL-8 level was also significantly increased in active BD. However, the serum IL-8 level was not significantly increased in patients with KD, compared with that of the controls, although neutrophilic vasculitis is a common finding in both BD and KD. An elevated serum concentration of S100A12 in KD patients has been shown to be correlated with inflammatory disease activity19,20. Also, in the present study, we demonstrated that elevated serum levels of S100A12 in BD patients were correlated with the BDCAF scores. However, one patient showed a paradoxical increase in S100A12, even after converting to the inactive phase by the treatment. It has been previously reported that serum S100A12 levels of some KD patients increased after intravenous immunoglobulin treatment19. Intracellular S100A12 levels of neutrophils in these KD patients were low19. Delayed elevation of the serum S100A12 may be caused by intracellular depletion, although we did not check the intracellular S100A12 levels of that BD patient. Other cytokines, such as TNF-α, may influence the delayed secretion of S100A12. To elucidate our hypothesis, both intracellular S100A12 staining and ex vivo neutrophil stimulation with TNF-α should be performed.
In conclusion, we demonstrated that S100A12 serum levels are increased in BD patients, and we performed immunohistochemical studies to reveal S100A12 expression patterns in specimens of erythema nodosum-like skin lesions from active BD patients. Our results indicate that S100A12 may have a pathogenic role related to neutrophil hyperactivity in BD patients, and therefore, serum levels of S100A12 may indicate disease activity in BD patients. However, because our pilot study only included ten BD patients, we need to further investigate the pathogenic role of S100A12 in BD in a larger sample of BD patients.
Figures and Tables
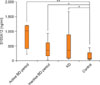 | Fig. 1Serum levels of S100A12 in the active and inactive period of Behçet's disease (BD), in Kawasaki disease (KD) and in healthy controls. The serum level of S100A12 was significantly increased in BD patients in the active period (**p<0.001), in BD patients in the inactive period (*p<0.05), and in KD patients (*p<0.05), compared with the serum levels of healthy controls. |
 | Fig. 2Comparison of serum S100A12 levels before and after treatment in Behçet's disease (BD) patients. Serum S100A12 levels from ten BD patients were significantly decreased eight weeks after systemic treatment (p=0.017). |
 | Fig. 3Comparison between the activity score and the serum S100A12 level in Behçet's disease (BD) patients. The serum S100A12 level significantly correlated with disease activity (spearman's coefficient=0.464, p=0.039). |
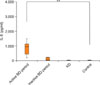 | Fig. 4Serum levels of interleukin (IL)-8 in the active and inactive periods of Behçet's disease (BD), in Kawasaki disease (KD), and in the healthy controls. Significantly increased serum IL-8 levels were found in the sera from active BD patients (**p<0.001), but not in the sera from inactive BD patients or KD patients, compared with the serum levels of healthy controls. |
 | Fig. 5Comparison of the serum IL-8 levels before and after systemic treatments in ten Behçet's disease (BD) patients revealed a decrease in the serum IL-8 level from baseline, although the change was not statistically significant (p=0.285). |
References
1. Zouboulis CC. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Adamantiades-Behçet disease. Fitzpatrick's dermatology in general medicine. 2007. 7th ed. New York: McGraw-Hill;1620–1626.
2. James DG. Behcet's syndrome. N Engl J Med. 1979. 301:431–432.
4. Kalayciyan A, Zouboulis C. An update on Behçet's disease. J Eur Acad Dermatol Venereol. 2007. 21:1–10.

5. Chun SI, Su WP, Lee S, Rogers RS 3rd. Erythema nodosum-like lesions in Behçet's syndrome: a histopathologic study of 30 cases. J Cutan Pathol. 1989. 16:259–265.

6. Demirkesen C, Tüzüner N, Mat C, Senocak M, Büyükbabani N, Tüzün Y, et al. Clinicopathologic evaluation of nodular cutaneous lesions of Behçet syndrome. Am J Clin Pathol. 2001. 116:341–346.

7. Jorizzo JL, Abernethy JL, White WL, Mangelsdorf HC, Zouboulis CC, Sarica R, et al. Mucocutaneous criteria for the diagnosis of Behçet's disease: an analysis of clinicopathologic data from multiple international centers. J Am Acad Dermatol. 1995. 32:968–976.

8. Takeno M, Kariyone A, Yamashita N, Takiguchi M, Mizushima Y, Kaneoka H, et al. Excessive function of peripheral blood neutrophils from patients with Behçet's disease and from HLA-B51 transgenic mice. Arthritis Rheum. 1995. 38:426–433.

10. Sahin S, Akoğlu T, Direskeneli H, Sen LS, Lawrence R. Neutrophil adhesion to endothelial cells and factors affecting adhesion in patients with Behçet's disease. Ann Rheum Dis. 1996. 55:128–133.

11. Rizzi R, Bruno S, Dammacco R. Behçet's disease: an immune-mediated vasculitis involving vessels of all sizes. Int J Clin Lab Res. 1997. 27:225–232.

12. Zouboulis CC, May T. Pathogenesis of Adamantiades-Behçet's disease. Med Microbiol Immunol. 2003. 192:149–155.

13. Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids. 2009. 36:381–389.

14. Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001. 69:986–994.
15. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999. 97:889–901.
16. Foell D, Wittkowski H, Hammerschmidt I, Wulffraat N, Schmeling H, Frosch M, et al. Monitoring neutrophil activation in juvenile rheumatoid arthritis by S100A12 serum concentrations. Arthritis Rheum. 2004. 50:1286–1295.

17. Boussac M, Garin J. Calcium-dependent secretion in human neutrophils: a proteomic approach. Electrophoresis. 2000. 21:665–672.

18. Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004. 50:3762–3771.

19. Ye F, Foell D, Hirono KI, Vogl T, Rui C, Yu X, et al. Neutrophil-derived S100A12 is profoundly upregulated in the early stage of acute Kawasaki disease. Am J Cardiol. 2004. 94:840–844.

20. Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, et al. S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet. 2003. 361:1270–1272.

21. Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003. 52:847–853.

22. Foell D, Hernández-Rodríguez J, Sánchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol. 2004. 204:311–316.

23. Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004. 344:37–51.

24. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001. 108:949–955.

25. Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002. 3:123–135.

26. International Study Group for Behçet's disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990. 335:1078–1080.
27. Freeman AF, Shulman ST. Kawasaki disease: summary of the American Heart Association guidelines. Am Fam Physician. 2006. 74:1141–1148.
28. Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000. 165:5814–5821.

29. Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001. 167:2879–2886.

30. Freire Ade L, Bertolo MB, de Pinho AJ Jr, Samara AM, Fernandes SR. Increased serum levels of interleukin-8 in polyarteritis nodosa and Behçet's disease. Clin Rheumatol. 2004. 23:203–205.

31. Gür-Toy G, Lenk N, Yalcin B, Aksaray S, Alli N. Serum interleukin-8 as a serologic marker of activity in Behçet's disease. Int J Dermatol. 2005. 44:657–660.
32. Durmazlar SP, Ulkar GB, Eskioglu F, Tatlican S, Mert A, Akgul A. Significance of serum interleukin-8 levels in patients with Behçet's disease: high levels may indicate vascular involvement. Int J Dermatol. 2009. 48:259–264.
33. Todaro M, Zerilli M, Triolo G, Iovino F, Patti M, Accardo-Palumbo A, et al. NF-κB protects Behçet's disease T cells against CD95-induced apoptosis up-regulating antiapoptotic proteins. Arthritis Rheum. 2005. 52:2179–2191.

34. Evereklioglu C, Er H, Türköz Y, Cekmen M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet's disease. Mediators Inflamm. 2002. 11:87–93.

35. Oztas MO, Onder M, Gurer MA, Bukan N, Sancak B. Serum interleukin 18 and tumour necrosis factor-alpha levels are increased in Behçet's disease. Clin Exp Dermatol. 2005. 30:61–63.

36. Raziuddin S, al-Dalaan A, Bahabri S, Siraj AK, al-Sedairy S. Divergent cytokine production profile in Behçet's disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatol. 1998. 25:329–333.
37. Akdeniz N, Esrefoglu M, Keleş MS, Karakuzu A, Atasoy M. Serum interleukin-2, interleukin-6, tumour necrosis factor-alpha and nitric oxide levels in patients with Behcet's disease. Ann Acad Med Singapore. 2004. 33:596–599.
38. Kose O, Stewart J, Waseem A, Lalli A, Fortune F. Expression of cytokeratins, adhesion and activation molecules in oral ulcers of Behçet's disease. Clin Exp Dermatol. 2008. 33:62–69.

39. Ahn SK, Choi EH, Lee SH, Lee S, Lee WS, Bong JP. Immunohistochemical study of Behcet's disease comparision of erythema nodosum-like lesion of Behcet s disease and erythema nodosum. J Wonju Coll Med. 1993. 6:214–223.
40. Ozoran K, Aydintuğ O, Tokgöz G, Düzgün N, Tutkak H, Gürler A. Serum levels of interleukin-8 in patients with Behçet's disease. Ann Rheum Dis. 1995. 54:610.
41. Zouboulis CC, Katsantonis J, Ketteler R, Treudler R, Kaklamani E, Hornemann S, et al. Adamantiades-Behçet's disease: interleukin-8 is increased in serum of patients with active oral and neurological manifestations and is secreted by small vessel endothelial cells. Arch Dermatol Res. 2000. 292:279–284.

42. Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992. 121:924–926.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download