Abstract
Background
The scales of bony fish represent a significant reservoir of calcium and calcification of the elasmoid scale is known to be associated with deposition of mineral crystals from the epidermis to dermis. However, little is known about the exact mechanisms of calcium deposition, mobilization and regeneration occurring in the zebrafish skin.
Objective
The purpose of this study was to investigate the expression of calcification-related molecular mediators in both the epidermis and dermis of the zebrafish (Danio rerio), using immunohistochemical study.
Methods
We examined the skin of zebrafish in four populations of different ages (i.e. 20 days post-fertilization (dpf), 35 dpf, 50 dpf, and the adult zebrafish), using several immunohistochemical markers, including bone morphogenetic protein 4 (BMP-4), β-catenin, osteocalcin, osteopontin and osteonectin.
In the bony fish, the scales are one of the elements constructing the dermal skeleton, along with the fin ray and the teeth. The elasmoid scale is the most common type of scale found in most of the bony fish, including the zebrafish (Danio rerio). In zebrafish, the scale formation starts from the region of the caudal peduncle in 25~30 day-old zebrafish, and it is observed in the epidermal-dermal boundary in the form of lamella. It is known that, in the process of scale development, the mineralization is progressive and the differentiation into a 3-layer structure (i.e. elasmodin or basal plate, the external layer, and the limiting layer, towards outside) occurs with deposition of mineralized crystals within scales. Then they work as an important reservoir to store calcium, but the mechanism of production, movement and deposition of calcium during the mineralization has not been clearly elucidated yet.
In this study, we investigated, via immunohistochemical staining, the expression level of the bone morphogenetic protein 4 (BMP-4), β-catenin, osteocalcin, osteopontin and osteonectin in both the epidermis and the dermis of the 3 weeks, 5 weeks, 7 weeks and 9 months post-fertilization zebrafish, and we attempted to elucidate the elements involved in the process of calcification in the elasmoid scale.
A total number of forty 3 week-old, 5 week-old, 7 week-old and 9 month-old zebrafish, (10 fish for each age), received from Zebrafish Organogenesis Mutant Bank (ZOMB) were used in our study.
They were fixated in 4% paraformaldehyde immediately after being anaesthetized with tricaine.
To observe the skin structures after the formation of scales, semi-thin sections were performed in 7 week-old and 9 month-old zebrafish. After washing the skin tissues fixated in 4% paraformaldehyde for 24 hours, they were dehydrated with 60, 70, 80, 90, 95% of ethanol for 10 minutes each, and with 100% ethanol for 10 minutes twice. Then they were substituted twice, for 15 minutes, with propylene oxide, were embedded in Epon812 (Polyscience, Warrington, PA). The embedded specimens were sliced into 1µm thick slices and were stained with 1% toluidine blue, to be examined under a light microscope.
For comparison, skin tissues were obtained from the abdomen area in 10 of each 3 week, 5 week, 7 week and 9 month old zebrafish and fixated in 4% paraformaldehyde for 24 hours. Then immunohistochemical staining was conducted with BMP-4, β-catenin, osteocalcin, osteopontin and osteonectin, using the commercial kit LSAB™+/horseradish peroxidase (HRP) system.
For immunohistochemical staining, the paraffin embedded tissues, microsectioned to a thickness of 3µm, were heated at 60℃ for 12 hours, were deparaffinized for 20 minutes with xylene, and were dehydrated for 5 minutes each in 100% ethanol and 75% ethanol. For antigen retrieval, the slides were dipped in 0.01 M citric acid buffer, boiled in the microwave for 4 minutes. The procedure was repeated 4 times, in cycles of of 30 seconds-boiling and 30 seconds-cooling in the microwave. Then the tissues were cooled for 1 hour at the room temperature, and processed with 3% hydrogen peroxide for 5 minutes at the room temperature, to inactivate the endogenous peroxidase. After reacting for an hour at room temperature, each primary antibody (i.e., biotinylated anti-rabbit, anti-mouse and anti-goat immunoglobulin) was added to the slides and reacted with the streptavidin HRP complex at room temperature, for 15 minutes (Table 1). Afterwards, color reaction was made with Dako substrate buffer 100 ul and DAB 2 ul, by controlling the reaction time, depending on the state of tissues. The slides were then examined after washing with hematoxylin for the contrast stain.
We investigated the intensity of staining in the epidermis and in the upper layer of the dermis by assessing the slides of at least 3 fish from each stage. We classified them into 'non-specific (-)', 'weakly positive (+)', 'moderately positive (++)' and 'strongly positive (+++)' by the intensity of staining to the relevant factors (Table 2).
Under the epidermis structured with 2~3 layers of cells, the dermis and the muscle structure were observed.
The scale tissue was not clearly observed with the light microscope (i.e. it can be seen only with the electronic microscope in the zebrafish older than 5 months).
Various sizes of epidermal cells were arranged in several layers in the epidermis and the collagen structure in the dermis consisted of different elements and densities, compared with the 7 week-old zebrafish. The scales, usually located between the epidermis and the dermis, were observed as a one-layer structure located very close to the basal plate of the epidermis.
A strong positive reaction in the entire epidermis of the 7 week-old zebrafish and moderate positivity in the epidermis of the 9 month-old zebrafish were observed, However, BMP-4 was not clearly expressed either in the epidermis or the dermis of animals younger than 5 weeks. In addition, the cells showing strong positivity were observed in the dermis located just below the epidermis.
β-catenin was not expressed in the epidermis and dermis of zebrafish younger than 5 week-old. Focal positivity was observed only in some cells of the epidermis of both 7 week-old and 9 month-old zebrafish.
The epidermis of 7 week-old and 9 month-old zebrafish showed weakly positive reactionto osteocalcin and the dermis was all negative.
The zebrafish (Danio rerio) belongs to the teleostes family is one of the intensively studied animal models because of its high homology with the human gene information and organ systems1. The epidermis of the zebrafish also shows high homology with the human epidermis, and several molecular genetic substances and mechanisms, including the retinol-binding protein 4 (rbp4) and apolipoprotein Eb (apoeb) were reported in both species in the process of development of the epidermis and the dermis of both the zebrafish and the upper vertebrates2.
The scale of the zebrafish can be found as a thin layer of collagen in the dermo-epidermal junction, and is known as an important reservoir of calcium, associated with the calcification which occurs during development3. However, studies on the mechanism of calcification are yet limited. The scales were observed in the outer layer of the dermis, paralleled with the skin surface, after 25~30 days post-fertilization. Elasmoid scales are formed by the deposition of the scale matrix produced from the scale-forming cells in the dermis3. During development, with the progression of mineralization, the elasmoid scale is differentiated into a 3-layer structures: the basal plate, the external layer and the limiting layer3-6. The process of mineralization starts from the external layer and progresses downwards6. The electron-dense mineral crystals spotted in the limiting layer areproduced by the basal layer cells of the epidermis and accumulate into the limiting layer through interaction between the epidermis and the dermis5. However, the exact molecular mechanism is still not clear.
In this study, the expression of calcification-related markers, previously reported in positive association with ectopic calcification, such as BMP-4, β-catenin, osteocalcin, osteopontin and osteonectin7-10, was assessed in the skin of zebrafish. Thus, we aimed to find out the type of molecular substances involved in the mineralization process in the skin of the zebrafish.
The bone morphogenetic protein (BMP) is the growth factor belonging to the transforming growth factor β (TGF-β) family and has been involved in the development of both ectopic calcification and regeneration of bone defects11-13. According to the results of this study, the epidermis and some of the dermis cells of the zebrafish express BMP-4 both before and after the age of 7 weeks, and are involved in the mineralization which proceeds in scale after 7 weeks. BMP-4 was stained most strongly in this study, and was therefore thought to play the major role among all investigated markers (i.e. BMP-4, β-catenin, osteocalcin, osteopontin and osteonectin). Furthermore, a few cells showing a strong positive reaction to BMP-4, compared to other cells, were observed in the dermis. It is uncertain whether these cells are scale-forming cells or other unrecognized cells participating in mineralization. Future studies are needed to investigate the exact character and function of these cells, including immunohistochemical study using electron microscopy.
The Wnt pathway induces the differentiation of osteocytes and osteoblasts, and correlation has been reported with the cardiovascular calcification, including the aortic valve, and with the ectopic calcification, such as the idiopathic cutaneous ossification7,14. In our study, however, contrary to previous results from ectopic calcification studies, β-catenin was weakly expressed only in some epidermal cells of the 9 month-old zebrafish and the reaction to β-catenin in the process of scale mineralization was not clearly observed.
Osteocalcin, osteopontin and osteonectin are bone-related protein antigens and are expressed by binding with calcium in the calcified tissues. Recently, they were found in calcified deposits and some endothelial cells of ectopic calcified tissue7-10,15, suggesting their role in the development of ectopic calcification. Our results indicate that one of the substances present in the epidermal cells of the zebrafish includes calcium crystals. Sire et al.5 conducted an electron microscope study and suggested that the mineral crystals deposited in the limiting layer were produced from the epidermis and then moved into the scaleThe strong expression of BMP-4 protein and deposition of the osteocalcin, the osteonectin and the osteopontin in epidermis observed in this study seem to support results from previous studies.
In conclusion, the BMP-4 was strongly expressed and the osteocalcin, the osteopontin and the osteonectin were weakly to moderately expressed in the epidermis of the zebrafish 7-weeks post-fertilisation. β-catenin was only observed in some epidermal cells. Our results indicate that the calcification-related proteins, including BMP-4, were expressed in the epidermis after scale formation. Given that the mineral crystals formed by the epidermal cells are deposited in the dermis through the epidermal-dermal interaction, our results suggest that calcification-related proteins, including BMP-4, may be associated with the deposition of mineral crystals in the process of calcification during the scale development. In addition, focal cells strongly expressing the BMP-4, the osteopontin and the osteonectin, were observed in the dermis. Further study is required to elucidate the exact properties of these dermal cells.
This study has a limitation, as it used only the light microscope, and additional studies, including electron microscope investigation combined with immunohistochemical staining, are needed to understand the exact characteristics and functions of the associated cells. Also, further investigation of RNA expression through whole mount in situ hybridization (WISH) is thought to be useful when the complete nucleotide sequences of calcium-related molecules in the zebrafish will become available. Our results may suggest the interaction between the epidermis and the dermis in the process of ectopic calcification in the zebrafish and may play a role in the understanding of the pathogenesis of ectopic calcification in both human skin and blood vessels.
Figures and Tables
Fig. 1
Semi-thin sections from the zebrafish skin. (A) 7 weeks. The 2 layer-thick epidermis (E) covers the dermis (D), which is composed of a relatively homogenous layer. (B) 9 months. Under the epidermis, well-developed collagen and elastic fibers (arrow) are seen in dermis. There is a thin, homogenous layer just below the epidermis, which is called the elasmoid scale (arrowhead) in zebrafish (Toluidine Blue stain, ×400).

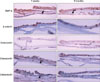
Fig. 2
Immunohistochemical stain for BMP-4, β-catenin, osteocalcin, osteopontin and osteonectin in 7 week-old and 9 month-old zebrafish skin. After 7 weeks, the epidermis (E) showed distinct positivity for BMP-4, osteopontin and osteonectin and some of the cells (arrows) in the dermis (D) also strongly expressed BMP-4, osteopontin and osteonectin (×400).
References
1. Detrich HW. Detrich HW, Westerfield M, Zon LI, editors. Overview of the zebrafish system. The zebrafish: biology in methods in cell biology. 1999. San Diego: Academic Press;5.
2. Tingaud-Sequeira A, Forgue J, André M, Babin PJ. Epidermal transient down-regulation of retinol-binding protein 4 and mirror expression of apolipoprotein Eb and estrogen receptor 2a during zebrafish fin and scale development. Dev Dyn. 2006. 235:3071–3079.

3. Sire JY, Akimenko MA. Scale development in fish: a review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio). Int J Dev Biol. 2004. 48:233–247.

4. Sire JY, Allizard F, Babiar O, Bourguignon J, Quilhac A. Scale development in zebrafish (Danio rerio). J Anat. 1997. 190:545–561.

5. Sire JY, Quilhac A, Bourguignon J, Allizard F. Evidence for participation of the epidermis in the deposition of superficial layer of scales in zebrafish (Danio rerio). J Morphol. 1997. 231:161–174.

6. Waterman RE. Fine structure of scale development in the teleost, Brachydanio rerio. Anat Rec. 1970. 168:361–379.
7. Kim SY, Choi HY, Myung KB, Choi YW. The expression of molecular mediators in the idiopathic cutaneous calcification and ossification. J Cutan Pathol. 2008. 35:826–831.

8. Urganus AL, Zhao YD, Pachman LM. Juvenile dermatomyositis calcification selectively displayed markers of bone formation. Arthritis Rheum. 2009. 61:501–508.

9. Marhaug G, Shah V, Shroff R, Varsani H, Wedderburn LR, Pilkington CA, et al. Age-dependent inhibition of ectopic calcification: a possible role for fetuin-A and osteopontin in patients with juvenile dermatomyositis with calcinosis. Rheumatology (Oxford). 2008. 47:1031–1037.

10. Davies CA, Jeziorska M, Freemont AJ, Herrick AL. Expression of osteonectin and matrix Gla protein in scleroderma patients with and without calcinosis. Rheumatology (Oxford). 2006. 45:1349–1355.

11. Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998. 16:247–252.

12. Mikhaylova L, Malmquist J, Nurminskaya M. Regulation of in vitro vascular calcification by BMP4, VEGF and Wnt3a. Calcif Tissue Int. 2007. 81:372–381.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download