Abstract
Background
For many years, the etiology of neonatal occipital alopecia (NOA) has been thought to be friction. It is recently clear that NOA is related to the physiological hair shedding.
Methods
Medical records of 240 postpartum patients who had been delivered between January 2006 and June 2007 at our institution were reviewed. Phone interviews with 193 respondents were conducted to investigate the actual conditions of NOA.
Results
NOA was present in 39 babies (20.2%). Univariate analysis showed that NOA was not associated with the baby's sleeping position, but was significantly associated with maternal parturition age, the delivery method, and the gestational age (p<0.05). In multiple logistic regression analysis, the risk of NOA was higher in the group younger than 35 years at parturition (OR, 3.86; 95% CI, 1.08~13.82), in the group not undergoing a Caesarean-section delivery (2.47; 1.09~5.60), and in the group delivered after 37 weeks of gestational age (3.36; 1.22~9.26).
Conclusion
The pregnancy-related factors, such as non-elderly gravida, non-Caesarean-section delivery, and enough gestational age, were associated with NOA. These findings support the recent theory that NOA is not an acquired alopecia, but a physiological condition, resulting from synchronized shedding of telogen hairs initiated in utero.
Neonatal occipital alopecia (NOA) is observed in the occiput of infants at 2~3 months after birth1-3. For a long time, it has been speculated that the scalp friction caused by the neonate's sleeping position might be a major etiology of NOA. It is recently clear, however, that NOA is a physiologic alopecia, progressing from the gestation period, rather than an acquired alopecia due to the physical friction1. Although NOA is relatively common, there have been few epidemiological reports of this condition, because it rarely shows severe symptoms that need treatment, and it improves spontaneously. Therefore, many physicians do not pay attention to this disease.
In the department of obstetrics of our institution, we investigated the prevalence of NOA in newborns, over a period of 18 months. In addition, we evaluated the risk factors of developing NOA.
A total of 338 newborns, who had been delivered between January 2006 and June 2007 in the department of obstetrics of our institution, were included in this study. Among them, for 240 subjects and their mothers we had data which could be assessed using medical charts, and 193 mothers, who were interviewed on the telephone, were ultimately enrolled in the current study.
Using the medical charts in 193 mothers, the parturition age, delivery method, parity, weight gain during pregnancy, gender of newborn, gestational age, and fetal birth weight were evaluated. The symptoms of NOA and of maternal telogen effluvium after childbirth were fully explained to respondents during the telephone interview. Then, the presence of both entities had to be individually identified. The sleeping posture of the infant was also checked. In cases of infants suffering from alopecia, the occurrence and the recovery time of the hair loss were assessed. While confirming the presence of NOA, cases with a past history of obstetric diseases, such as caput succedaneum or cephalhematoma, cases in which the hair loss occurred in areas other than the occiput, and cases in which alopecia was persistently present for more than one year after birth, were all excluded from the current analysis. Through a factor and reliability analysis in each category, the elimination and purification of variables were performed and all the variables were dichotomized. Based on the high-risk parturition age of 35 years, mothers were divided into two groups4: the group of mothers aged 21 to 34 years and the group ≥35 years. In accordance with the delivery method, mothers were also divided into two groups: the first group with non-Caesarean-section (non-C-sec) delivery (i.e. normal delivery, induction delivery or forceps delivery) and the group with the Caesarean-section (C-sec) delivery under general anesthesia. Depending on the parity, mothers were divided into primipara and multipara. Based on the cut-off value of mean weight gain of 12 kg during pregnancy4, mothers were also divided into two groups: the group of ≥12 kg and the group of <12 kg. Similarly, in regard to the gestational age and the fetal birth weight, as criteria for determining prematurity5, fetuses were divided into groups: the group of ≥37 weeks gestational age and <37 weeks, and the group of ≥2.5 kg at birth and <2.5 kg. Based on their sleeping posture, fetuses were also divided into two groups: the supine position group and the prone position group.
Statistical analysis was performed using the SPSS for Windows software, Version 17.0, (SPSS Inc, Chicago, IL, USA). For comparisons between the selected groups, we used either the χ2 test or the Fisher's exact test (as appropriate) for categorical variables. Factors associated with NOA were identified using the multiple logistic regression method. A p-value less than 0.05 indicated a statistically significant difference.
Table 1 details the subject characteristics and research findings. The mean parturition age of 193 mothers was 32.5 years (range: 21~44 years). Among the 193 babies, there were 94 male (48.7%) and 99 female (51.3%). The mean gestational age was 37 weeks and the mean weight at birth was 2.9 kg. The number of subjects with NOA was 39 (20.2%). After birth, the mean occurrence time and the mean restoration time of NOA were 2.8 months and 6.5 months, respectively.
In newborns delivered from mothers aged 34 years or younger, 36 cases (92.3%) of alopecia were reported (p=0.012). In newborns delivered using non-C-sec methods, 27 cases (69.2%) of alopecia were developed (p=0.022) (Table 2). Alopecia was seen in 21 cases (53.8%) of primipara, 31 cases (79.5%) of mothers whose weight increased by more than 12 kg during pregnancy and 29 cases (74.4%) of mothers who developed a telogen effluvium after childbirth. However, the differences found in these results were not statistically significant.
Of 39 newborns who developed alopecia, 18 babies were male (46.2%) and 21 babies were female (53.8%). The male-to-female ratio was 0.86:1.17, but there was no significant difference in the sex distribution. In the group with a gestational age of ≥37 weeks, 29 cases (74.4%) of alopecia were reported. This indicates that the incidence of alopecia was significantly increased in the group in which the gestational age was relatively older (p=0.003) (Table 2). In the group of newborns with the birth weight greater than 2.5 kg and in those sleeping in the supine position, there were 30 cases (76.9%) and 37 cases (94.9%) of alopecia, respectively. However, the differences did not show any statistical significance.
To ascertain the independent factors assumed to have a significant relationship with NOA, a multivariate analysis was performed on the variables showing a p-value <0.2 on univariate analysis. The results showed independent associations between NOA and parity, weight gain during pregnancy, telogen effluvium of the mother, fetal gender, gestational age, fetal weight at birth, and sleeping position of the infant. Results were statistically significant for mothers' delivery age ≤34 years (p=0.038), those with delivery via the non-C-sec method (p=0.039), and infants with the gestational age ≥37 weeks (p=0.019) (Table 3).
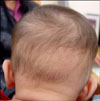
NOA is a type of non-scarring, localized alopecia, which occurs temporarily on the occiput of 2~3 months-old babies. Since NOA was first reported by Brocq2 in 1907, this disease has showed a prevalence of 9~12%1 and has occurred more prevalently in Caucasian infants3,6,7. In a band-like shape or oval alopecic patch, its lower margin is sharp and consists of a band of hair extending to the nape, but the upper limit is merged with the hair of the vertex in a less marked manner (Fig. 1). The cause of this entity is thought to be a combination of physiologic telogen effluvium and, possibly, localized pressure owing to the neonate's sleeping position8.
In the past, the occipital hair loss of the infant was commonly suspected to be due to the friction between the pillow and the scalp. Accordingly, there were many instances when infants were laid on their face, and these situations resulted in increased possibility for sudden infant death syndrome (SIDS). Given this background, in 1992, the American Academy of Pediatrics (AAP) recommended that infants should sleep in a supine position, rather than a prone position, to reduce the occurrence of SIDS9,10. Cutrone and Grimalt1 examined the prevalence of NOA prior to and following the AAP guidelines, and suggested that there was no significant correlation between the sleeping position of infants and the occurrence of NOA. These results seem to be supported by our data showing that the incidence rates of NOA were higher, but not statistically significantly different in the group of infants sleeping in the supine position.
In this study, the incidence of NOA was significantly higher in cases with mothers' delivery age ≤34 years and non-C-sec delivery, compared with otherwise groups. In contrast to the previous reports, describing that alopecia areata often occurs in premature infants11-13, the incidence of NOA in our study was significantly increased in the group with a gestational age ≥37 weeks than in the group showing prematurity. Taken together, it seems that NOA occurs more often in a baby born from a mother experiencing either normal or low-risk gravid and laboring state. This finding suggests that NOA is correlated with both the gestation and the delivery periods, rather than the postnatal period, and is more affected by a normal pregnancy state, rather than an unfavorable state. This supports the hypothesis that NOA is a physiologic process of hair shedding developed since gestation.
The hair development on the fetal scalp begins at 9~12 weeks of gestation and the whole scalp is covered with anagen hair by 18~20 weeks of gestation7,14,15. Although the hair roots go through the catagen and then the telogen phases in a progressive manner, i.e. from frontal to parietal areas at 26~28 weeks of gestation, those in the occipital area are not involved in this process and remain in the anagen phase due to an operative signal, delaying the onset of telogen until close to term3. Later, these hairs abruptly go into the telogen phase and are inevitably shed 8~12 weeks after birth3. If the pregnant process does not progress properly, we suggest that the signal may be interrupted, leading to absence of NOA. Based on changes in different phases of the follicular cycle, Headington16 recently classified the telogen effluvium into five functional types. Of them, he proposed the "delayed anagen release", which means that some follicles remain in prolonged anagen until they abruptly enter telogen. He also suggested that the telogen effluvium of the postpartum woman is the most notable alopecia associated with delayed anagen release. In the current study, there was no significant correlation between NOA and the maternal telogen effluvium. Therefore, although they have a similar mechanism for hair loss and a tendency to occur around the same time, these conditions are presumed to develop independently, i.e. without a common operative signal.
Meanwhile, NOA could not be necessarily identified in all infants. Cutrone et al. suggested that a sufficient amount of anagen hair might replace the alopecia site after some telogen hair shedding occured in some infants. Moreover, since there are mild symptoms and consequent spontaneous resolution in most cases of NOA, it is assumed that parents are not aware of this disease entity and forget about it. As mentioned earlier, the incidence of NOA has been reported to be relatively higher in fair-complexioned (Caucasian) neonates. In children with a dark complexion, the onset of the change, i.e. delayed anagen release, is postponed, so most hair roots are still in anagen at birth, and the mean diameter of the hairs is greater than in fair-complexioned neonates3,6,7. For these reasons, this type of alopecia might be comparatively more noticed in the fair neonates. Although the prevalence of NOA in Korean babies' was relatively higher in this study than in the previous Caucasian study1, this might be due to differences in the research methods and in the sample size.
The important differential diagnoses of NOA to be considered are pressure alopecia, the halo scalp ring, and alopecia areata1.
There are some limitations to our study. First, the presence and extent of NOA were assessed by the self-report method. In general, a diagnosis of telogen effluvium is made based on the personal history or clinical manifestation and may be assisted through several tests, such as the hair pulling test, trichogram, and scalp biopsy17,18. The diagnosis of NOA in this study was not made through clinical assessment or test by physicians. However, the diagnosis might be reliable to some extent, because mothers were given full explanation by dermatologists about the characteristics of NOA and the differentiated diseases, such as caput succedaneum, cephalhematoma, and the underlying tumor. Second, since this was a retrospective study, there might be recall and selection bias from the study subjects. Nonetheless, the results from this study would be of significance, in that it first clarified the relevant factors of NOA. To amend the above limitations, further large-scale prospective studies should be warranted in a universal group of patients who are objectively diagnosed. In conclusion, the postnatal factors, including the baby's sleeping position, are not associated with NOA, while the favorable pregnancy-related factors, such as non-elderly gravida, non-C-sec delivery, and enough gestational age, are associated with NOA. These results support the theory that NOA is not an acquired alopecia, but a physiological condition resulting from synchronized shedding of telogen hairs initiated in utero. Therefore, it is important to assure parents that NOA is, by and large, not associated with the sleeping posture of infants and is a self-limited disease.
Figures and Tables
References
1. Cutrone M, Grimalt R. Transient neonatal hair loss: a common transient neonatal dermatosis. Eur J Pediatr. 2005. 164:630–632.

2. Brocq L. Traite elementaire de dermatologie pratique. 1907. Vol. 1. Paris: Octave Doin;358.
3. Rogers M. Eichenfield LF, Frieden IJ, Esterly NB, editors. Hair loss in the neonate. Textbook of neonatal dermatology. 2001. 1st ed. St. Louis: Mosby;494.
4. Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Williams obstetrics. 2010. 23rd ed. New York: McGraw-Hill;189–214.
5. Stoll BJ, Adams-Chapman I. Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. The high risk infants. Nelson textbook of pediatrics. 2007. 18th ed. Philadelphia: WB Saunders;698–711.
6. Barman JM, Pecoraro V, Astore I, Ferrer J. The first stage in the natural history of the human scalp hair cycle. J Invest Dermatol. 1967. 48:138–142.
8. Chang MW, Orlow SJ. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Neonatal, pediatric, and adolescent dermatology. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;935–955.
9. American Academy of Pediatrics AAP Task Force on Infant Positioning and SIDS: Positioning and SIDS. Pediatrics. 1992. 89:1120–1126.
10. Gibson E, Cullen JA, Spinner S, Rankin K, Spitzer AR. Infant sleep position following new AAP guidelines. American Academy of Pediatrics. Pediatrics. 1995. 96:69–72.
12. Bardazzi F, Neri I, Raone B, Patrizi A. Congenital alopecia areata: another case. Dermatology. 1999. 199:369.

13. Crowder JA, Frieden IJ, Price VH. Alopecia areata in infants and newborns. Pediatr Dermatol. 2002. 19:155–158.

14. Pinkus H. Montagna W, Ellis RA, editors. Embryology of hair. The biology of hair growth. 1958. New York: Academic Press;1–32.

15. Olsen EA. Harper J, Oranje A, Prose N, editors. Hair disorders. Textbook of pediatric dermatology. 2006. 2nd ed. Oxford: Blackwell Publishing;1753–1782.

18. Sperling LC. Hair and systemic disease. Dermatol Clin. 2001. 19:711–726. ix




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download