Abstract
Background
Several differences in basal cell carcinomas (BCCs) were found, according to the ethnic group; for example, pigmented BCCs was more common in Asian or Hispanic patients. However, there are few reports on the subclinical extension of the BCC in Asian patients.
Objective
The aim of this study was to evaluate the subclinical infiltration of the basal cell carcinoma in Asian patients.
Methods
All patients with BCC who visited the department of dermatology at Korea University Ansan Hospital were treated with Mohs micrographic surgery. In 81 patients, 83 tumors of BCC were completely eradicated by Mohs micrographic surgery (MMS) from April 2001 to August 2008, and were reviewed in this study. Information recorded included the total margin and the number of stages of Mohs micrographic surgery, anatomic location, tumor size, presence of pigmentation, clinical type, and pathological subtype. We divided the clinical types into nodular, ulcerated, and pigmented, and the pathological types into nodular, micronodular, morpheaform, and adenoid. The BCC was of pigmented type if pigmentation covered more than 25% of the tumor, regardless of whether pigmentation was distinct, or if there was apparent pigmentation that covered more than 10% of the tumor.
Results
The nose and cheek were the most common sites requiring more than one stage of surgery. In tumors smaller than 1 cm, 91.7% required only one stage of excision, compared with 60.6% in tumors larger than 1 cm. More than two Mohs stages were required in 25% of non-ulcerated BCCs and in 46.2% of ulcerated BCCs. Sixty eight percent of pigmented BCCs required only one stage of Mohs micrographic surgery. In cases of non-pigmented BCCs, only 45% required one Mohs stage. More than one Mohs stage was required in 19.2% of non-aggressive BCCs and in 42.9% of aggressive BCCs.
Conclusion
Subclinical infiltration differed between the two groups according to the size of the BCC (1 cm threshold) and most of the BCCs were located in the head and neck area. Considering this result, indication for MMS can be extended for BCCs larger than 1 cm in Asian patients. Ulcerated BCCs required more Mohs stages than non-ulcerated BCCs. Pigmented BCCs might show lesser subclinical infiltration than non-pigmented BCCs. Aggressive pathological subtypes showed more subclinical infiltration than the non-aggressive types; however, after evaluation of the border that was excised with MMS, mixed histologic types were found to be more frequent than generally accepted. Therefore, we consider that, when planning surgery, dermatologists should not place too much confidence in the pathologic subtypes identified by biopsy.
Basal cell carcinoma (BCC) is the most common skin cancer, with increasing annual incidence in the Asians1,2 population. Although Mohs micrographic surgery (MMS) is the treatment of choice for BCC, the recurrence rate of BCC, even with MMS, is about 1~2% for the primary BCC, and 4~5% for the recurrent BCC, The cause of recurrence may be the subclinical histologic extension of the tumor, beyond the clinical margin. It is the reason why we need to understand the subclinical infiltration of BCC, to increase the rate of complete excision by taking that pattern into account, along with several other factors affecting incomplete excision; e.g. racial differences, tumor size, tumor site, and histologic subtype.
Several differences in BCCs were found, according to the ethnic group; for example, pigmented BCCs were more common in Asian or Hispanic patients3,4. Until now, there have been few reports evaluating the subclinical margin of BCC in Asian patients. If there are differences in the subclinical extension and associated factors between Asians and Caucasians, there would be some differences in the recurrence of BCC, even with the same treatment. Therefore, we examined the clinical and histopathologic characteristics and the subclinical infiltration of 83 cases of BCC, completely excised by MMS at the Korea University hospital, from April 2001 to August 2008.
All patients with BCC who visited the department of dermatology at Korea University Ansan Hospital were treated with MMS, except for several patients who did not want surgical treatment. Of a total of 81 patients, 83 tumors of BCC that were completely eradicated by MMS from April 2001 to August 2008 were reviewed in this study.
The recorded information included age, sex, total margin and the number of stages of MMS, anatomic location, tumor size, presence of pigmentation, clinical type, and pathological subtype. We excised the tumor with a 2 mm margin from the tumor border, assessed using loupe magnification. If a tumor was present on histologic examination, we excised additional skin with a 2 mm margin for each stage of MMS. Pathologic subtypes were categorized into four subtypes, i.e. nodular, micronodular, morpheaform, and adenoid. If one tumor had several pathological subtypes, all subtypes were counted separately, therefore all pathological subtypes were more than all tumor cases (i.e. 120 vs 83). Nodular and adenoid subtypes were grouped into non-aggressive types and the remaining two subtypes into aggressive types. The BCC was designated as pigmented if pigmentation covered more than 25% of the tumor, whether pigmentation was distinct, or if there was apparent pigmentation that covered more than 10% of the tumor. The number of stages with other variables mentioned above was preliminarily analyzed with the χ2 test. A p-value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS software version 12.0.
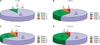
Clinical and pathological tumor characteristics are shown in Table 1. The average total margin and the number of stages required for complete tumor excision were 3.08 mm and 1.46 stages, respectively. Most of the tumors were located on the head and neck (79 cases, 95.2%). The nose and cheek were the most common sites requiring more than one stage surgery; however, considering the incidence, anatomic sites are not the factor affecting the number of MMS stages (Table 2). Most BCCs are 1~2 cm in size. No statistical difference in the MMS stage was observed between the two groups (tumors <2 cm vs. tumors ≥2 cm). However, in tumors smaller than 1 cm, 91.7% required only one stage of excision, compared with 60.6% of tumors larger than 1 cm (p<0.037) (Fig. 1A). More than one stage was required in 46.2% of ulcerated BCCs and in 25% of non-ulcerated BCCs, and there was a significant difference between the two groups (Table 1). Seventy two cases (86.7%) had pigmentation. No statistically significant difference in the number of stages was identified between pigmented and non-pigmented BCCs (Table 1). Pathological subtypes according to pigmentation are shown in Fig. 2. Nodular types were more than half of the pigmented BCCs; however, in non-pigmented BCCs, 47% were micronodular. Mixed types were 60% of all cases. While 100% of non-aggressive types had clinically visible pigmentation, only 82.1% of aggressive types had pigmentation. 42.9% of aggressive pathologic types required more than 2 stages; however, only 19.2% of non-aggressive types required more than 2 stages. This difference was statistically significant (p<0.037) (Table 1).
The purpose of the present study was to identify various factors associated with subclinical extension of the BCC in Asians.
In our study, 30.9% of patients with BCC were in their sixties, followed by seventies (27.2%), and fifties (18.5%). Female patients were more than male patients (58% vs. 42%). Ninety five point two percent of BCCs developed on the head and neck, and only 4% were observed on the trunk and extremities. The nose and the cheek area were the most common sites requiring more than one stage surgery, followed by the periorbital area and forehead; however, considering the incidence, there was no statistically significant difference between these sites (i.e. nose 57%, cheek 50%, the periorbital area 24%, the forehead 31%).
Average stage and margin required for complete tumor excision were 1.46 and 3.04 mm, respectively. In 65% of patients, only 1 stage was needed. Kim et al.5 reported that 55% of BCCs required only one stage. Leibovitch et al.6 reported that the average number of stages for complete excision was 1.73. In reports presented by Smeets et al, only 25% of BCCs required only one stage7. The reason for this difference between studies may be due to inconsistency in subjects' selection for MMS, in tumor margins decided without a magnifying glass and in the amount of surgical margin excised at the first stage.
A number of reports have shown a higher rate of incomplete excision in tumors larger than 2 cm, compared to smaller ones; however, the difference was not statistically significant8-10. In our study, the percentage of tumors requiring only one Mohs stage was 68% in tumors smaller than 2 cm and 55% in those larger than 2 cm; however, there was no statistically significant difference between the two groups.
When a smaller size was considered (tumors <1 cm, tumors ≥1 cm), the difference became significant (i.e. 92% in tumors <1 cm, and 60% in tumors ≥1 cm). Several guidelines divide tumors into two main groups, i.e. the low risk and the high risk group (tumors <2 cm, tumors >2 cm)11-13. A tumor larger than 2 cm is an indication for MMS14; however, according to some reports, the subclinical extension or recurrence rate differed significantly between the two groups (tumors <1 cm, tumors ≥1 cm)15. According to the National Comprehensive Cancer Network (NCCN) guidelines, the high risk is defined by a tumor larger than 10 mm in the middle risk body area or 6 mm, in the high risk body area16. Considering that most of the tumors in our study were found on the head and neck, which are middle or high risk body areas, our results showing significant differences between the two groups of tumors (i.e. larger and smaller than 1 cm) corresponded to the NCCN guidelines. Therefore, in Asian patients, the indication for MMS could be extended for tumors larger than 1 cm, with consideration for several points. First, MMS has the advantage of complete evaluation of all margins. Second, margin positive rate was reported to be about 20%, if tumors were excised with a narrow margin of 1~3 mm without checking free margin17,18. Third, in our study, only 8% of tumors below 1cm required more than one excision.
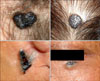
In our study, 78% of basal cell carcinomas showed pigmentation, and this result was similar to those of other studies conducted in Asians3,19. The percentage of pigmented BCC in our study was remarkably higher than that of Caucasians, showing only about 10% of BCC pigmentation20,21. We studied the number of stages according to the pigmentation of the BCC. The complete excision rate at one stage was 68.1% in pigmented BCC and 45.5% in non-pigmented BCC. Hornblass and Stefano22 also reported that the complete excision rate was higher in pigmented BCC than in non-pigmented ones. Maloney et al.20 presented the same opinion in their reports, in which only one of 40 pigmented BCCs showed positive margins at one stage of MMS. In our study, the incidence of subclinical extension was lower in pigmented BCC than in non-pigmented BCC, although there was no statistically significant difference between the two types. The clinical spectrum of pigmented BCCs was broad, ranging from BCCs showing speckled pigmentation in small areas to those showing total, even pigmentation. Evaluation of the subclinical extension of the BCC according to the individual pattern of pigmentation, rather than simply grouping into pigmented or non pigmented BCCs, would be preferable. We found some characteristics of pigmented BCCs. Thus, most pigmented BCCs showing even pigmentation were excised completely at one stage (Fig. 3A). If there was a bluish hue surrounding the tumor, that tumor usually required more than one excision, despite having overall even pigmentation (Fig. 3B).
Fifty three point eight percent of BCCs with clinical ulcer and 75% of BCCs without clinical ulcer were completely excised at one stage, and the difference was statistically significant (Table 1). Therefore, it seems that ulceration could indicate the aggressiveness of BCCs.
The percentage of BCCs requiring only one MMS stage was 57.1% and 80.8% in the aggressive type and the non-aggressive type, respectively. This result was similar to those of several previous reports15,16,23.
In our study, the BCCs with mixed pathologic type were 60% of all cases. This percentage was higher than previously reported1,24. This result may be attributed to the fact that we studied the border of the BCC after removal of the tumor with MMS. These lesions may have been diagnosed as one certain type of BCC with punch biopsy. This point supports the necessity of MMS for BCC.
In conclusion, in our study, the average stage and margin required for complete tumor excision were 1.46 and 3.04 mm, respectively. Subclinical infiltration differed between the two groups according to the size of the BCC (smaller than 1 cm vs. larger than 1 cm) and most BCCs were located in the head and neck area. Considering this result, in Asian patients, the indication for MMS can be extended for BCCs larger than 1 cm. Ulcerated BCCs required more Mohs stages than non-ulcerated BCCs. Our results suggest that pigmented BCCs might show lesser subclinical infiltration than non-pigmented BCCs. However, as the degree of pigmentation varies largely it would be better to evaluate the pigmented BCCs according to the individual pattern of pigmentation, rather than simply considering the existence of pigmentation. The aggressive pathologic subtypes show more subclinical infiltration than the non-aggressive types; however, after evaluation of the border that was excised with MMS, mixed histologic types were found to be more frequent than generally accepted. Therefore, when planning surgery, dermatologists should not place too much confidence in the pathologic subtypes identified by biopsy.
Figures and Tables
Fig. 2
Pathologic subtypes of basal cell carcinoma according to pigmentation. Nod: nodular, Mor: morpheaform, Mic: micronodular, Ade: adenoid.

References
1. Song ES, Cho BK, Kim SY, Kim SN, Suh KS, Son SJ, et al. A clinicopathological study of basal cell carcinoma in Korean patients. Korean J Dermatol. 2000. 38:762–771.
2. Ishihara K, Saida T, Otsuka F, Yamazaki N. Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008. 13:33–41.

3. Kikuchi A, Shimizu H, Nishikawa T. Clinical histopathological characteristics of basal cell carcinoma in Japanese patients. Arch Dermatol. 1996. 132:320–324.

4. Bigler C, Feldman J, Hall E, Padilla RS. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996. 34:751–752.

5. Kim EJ, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. A clinicopathological study of facial basal cell carcinoma treated by Mohs micrographic surgery. Korean J Dermatol. 2006. 44:721–726.
6. Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia I. Experience over 10 years. J Am Acad Dermatol. 2005. 53:445–451.

7. Smeets NW, Kuijpers DI, Nelemans P, Ostertag JU, Verhaegh ME, Krekels GA, et al. Mohs' micrographic surgery for treatment of basal cell carcinoma of the face--results of a retrospective study and review of the literature. Br J Dermatol. 2004. 151:141–147.

8. Bhatti AZ, Asif S, Alwan M. Factors affecting incomplete excision of nonmelanoma skin cancers in New Zealand. Ann Plast Surg. 2006. 57:513–516.

9. Bøgelund FS, Philipsen PA, Gniadecki R. Factors affecting the recurrence rate of basal cell carcinoma. Acta Derm Venereol. 2007. 87:330–334.

10. Kumar P, Watson S, Brain AN, Davenport PJ, McWilliam LJ, Banerjee SS, et al. Incomplete excision of basal cell carcinoma: a prospective multicentre audit. Br J Plast Surg. 2002. 55:616–622.

11. Telfer NR, Colver GB, Morton CA. British Association of Dermatologists. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008. 159:35–48.

12. Sterry W. European Dermatology Forum Guideline Committee. Guidelines: the management of basal cell carcinoma. Eur J Dermatol. 2006. 16:467–475.
13. Netscher DT, Spira M. Basal cell carcinoma: an overview of tumor biology and treatment. Plast Reconstr Surg. 2004. 113:74e–94e.

14. Carucci JA, Leffell DJ. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Basal cell carcinoma. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1036–1042.
15. Batra RS, Kelley LC. Predictors of extensive subclinical spread in nonmelanoma skin cancer treated with Mohs micrographic surgery. Arch Dermatol. 2002. 138:1043–1051.

16. National Comprehensive Cancer Network. Basal cell and squamous cell skin cancers. NCCN Clinical Practice Guidelines in Oncology. 2009.
17. Hsuan JD, Harrad RA, Potts MJ, Collins C. Small margin excision of periocular basal cell carcinoma: 5 year results. Br J Ophthalmol. 2004. 88:358–360.

18. Kimyai-Asadi A, Alam M, Goldberg LH, Peterson SR, Silapunt S, Jih MH. Efficacy of narrow-margin excision of well-demarcated primary facial basal cell carcinomas. J Am Acad Dermatol. 2005. 53:464–468.

19. Tan WP, Tan AW, Ee HL, Kumarasinghe P, Tan SH. Melanization in basal cell carcinomas: microscopic characterization of clinically pigmented and non-pigmented tumours. Australas J Dermatol. 2008. 49:202–206.

20. Maloney ME, Jones DB, Sexton FM. Pigmented basal cell carcinoma: investigation of 70 cases. J Am Acad Dermatol. 1992. 27:74–78.

21. Betti R, Gualandri L, Cerri A, Inselvini E, Crosti C. Clinical features and histologic pattern analysis of pigmented basal cell carcinomas in an Italian population. J Dermatol. 1998. 25:691–694.

22. Hornblass A, Stefano JA. Pigmented basal cell carcinoma of the eyelids. Am J Ophthalmol. 1981. 92:193–197.

23. Farhi D, Dupin N, Palangié A, Carlotti A, Avril MF. Incomplete excision of basal cell carcinoma: rate and associated factors among 362 consecutive cases. Dermatol Surg. 2007. 33:1207–1214.

24. Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma. Study of a series of 1039 consecutive neoplasms. J Am Acad Dermatol. 1990. 23:1118–1126.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download