Abstract
Background
Although photodynamic therapy (PDT) is widely performed for acne, little is known about its exact therapeutic mechanism.
Objective
We aimed to estimate the efficacy and safety of PDT on acne and to discover its mode of action.
Methods
We performed PDT on 12 patients with mild to moderate acne. The clinical efficacy was assessed by counting the acne lesions and measuring the sebum secretion before and after PDT. In addition, we took biopsy samples from the peri-lesional areas before and after 3-session of PDT. To examine the degree of apoptosis of the sebaceous follicles, TUNEL assay was performed. To investigate the changes of toll-like receptor (TLR)-2 and TLR-4 expression after PDT, immunohistochemical stainings were also carried out. Finally, we performed TUNEL assay using the cultured sebocytes to confirm the apoptosis of sebocytes in vitro after PDT.
Results
There was a significant reduction in the number of inflammatory acne lesions after PDT, compared to baseline (p<0.05). Sebum excretion significantly decreased 2 weeks after the first PDT session except for one patient (p<0.05). The TUNEL positive cells in the peri-lesional sebaceous glands after PDT markedly increased, compared with those of before PDT. A decrease in TLR-2 and TLR-4 expression by sebaceous glands and epidermis after PDT was 50% and 30%, respectively.
Acne is one of the most common diseases in adults as well as in adolescents which may cause post-inflammatory pigmentation and permanent scarring1,2. It is a multi-factorial disease originating from sebaceous follicles. Its widely recognized pathophysiology is based on four factors: follicular hyperkeratinization, colonization of Propionibacterium acnes, increased sebum production, and inflammation3. Recently, toll-like receptors (TLRs) which play a role in innate immune response, especially TLR-2 and TLR-4, have been shown to be related to P. acnes in acne inflammation4.
Conventional treatments for acne, including topical agents, chemical peelings with β-salicylic acid or glycolic acid, and oral medications such as antibiotics and isotretinoin, are widely prescribed despite their associated problems. Although isotretinoin is an effective therapeutic agent for intractable cases, some patients refuse its oral prescription because of its complication such as teratogenicity and cheilitis. In particular, childbearing age patients should avoid this medication. Another problem is an increasing tendency of resistance of P. acnes to antibiotics5. Thus avoidance of long-term usage of antibiotics is desirable. Furthermore, there are some intractable patients who do not respond to conventional treatment modalities; another therapeutic modality is therefore required. The recent introduction of photodynamic therapy (PDT) has provided dermatologists with another useful treatment modality for acne patients.
PDT with topical aminolevulinic acid (ALA) was originally used to treat actinic keratoses and superficial basal cell carcinomas6. There has recently been a growing body of evidence that PDT is also effective for the treatment of acne. The exact mechanisms are not well known, but this approach is based on the fact that porphyrins accumulate in the epidermis and hair follicles, with some preference in the sebaceous glands, the target organ of acne7. After taken by the pilosebaceous units, through the heme metabolic pathway, ALA is metabolized to protoporphyrin IX (Pp IX), a potent photosensitizer. With visible light exposure, accumulated Pp IX is excited into the triple state, which reacts with oxygen to produce singlet oxygen and free radicals. This induces photodamage to the sebaceous glands8.
In this study, we performed topical ALA-PDT in 12 patients with mild to moderate acne, aiming to estimate the efficacy and safety of PDT on acne and to discover its mode of action.
After obtaining informed consent, 12 patients of both sexes with mild to moderate acne vulgaris on their faces were enrolled in this study. Patients were excluded if they were prescribed and had used systemic retinoids or antibiotics in the past 4 weeks or had a history of keloid or a photosensitive disorder. Pregnant or lactating women were also excluded.
Baseline clinical evaluations were performed, including photography, sebum output (SO) measurement and inflammatory and non-inflammatory acne lesion count. The inflammatory lesions were defined as the summation of pustules, erythematous papules and nodules. White comedones and black comedones were regarded as non-inflammatory lesions. We measured SO with the sebumeter (SM810®, Courage+Khasaka Electronic GmbH, Köln, Germany) from both cheeks three times and calculated their average. Before applying ALA, the face was cleansed with 70% isopropyl alcohol after self washing with soap. Then, in a darkroom, we applied 20% topical ALA in a hydroalcoholic vehicle (Levulan®, DUSA Pharmaceuticals Inc., Wilmington, MA, USA) onto the whole face and left it under occlusion with a plastic wrap for an hour. After self cleansing and cleansing with alcohol, patients were irradiated with a narrow band LED (Omnilux®; Waldmann Medizintechnik, Villingen-Schwennings, Germany, 633±3 nm), with a light intensity of 105 mW/cm2 and light doses of 20~30 J/cm2, 10~15 cm away from their face. After each treatment, they were asked to keep away from sun exposure for 48 hours if possible. When absolute sun avoidance was not possible within 48 hours after the PDT, they were educated to apply enough amounts of sunscreen hourly. They were treated three times, every two weeks, and were asked to visit monthly for 3 months thereafter. At each visit and follow-up, we counted the number of acne lesions. Also, patients were photographed and evaluated for clinical alteration and SO.
At the end of the study, patients were questioned about the adverse effects and demerits of PDT treatment on acne.
Punch biopsy specimens (3 mm) were taken from the perilesional acne lesion before the start of therapy and immediately after the last PDT session from each patient. The samples were fixed with 4% paraformaldehyde (PFA), embedded in paraffin and then sections of 5µm were made on silane-coated micro slides (Muto-glass, Tokyo, Japan). The de-paraffinized skin sections were stained with hematoxylin and eosin staining.
The samples were fixed with 4% PFA, embedded in paraffin and then sections of 5µm were made on silane-coated micro slides (Muto-glass, Tokyo, Japan). The de-paraffinized skin sections were stained with immunohistochemistry. The sections were rinsed with distilled water and endogenous peroxidase activity was blocked with 3% hydrogen peroxide (H2O2) solution diluted with methanol. After rinsing with phosphate-buffered saline (PBS), they were incubated with 0.01M citrate buffer (pH 6) at 105℃ for 15 minutes and then, at room temperature for 40 minutes. Then, nonspecific antigen-antibody reactions were inhibited by 1 hour treatment with 2.5% normal horse serum (R.T.U. Vectastatin® Universal ABC Elite kit, Vector Laboratories, Inc., Burlingame, CA, USA). The sections were left overnight at 4℃ with purified rabbit polyclonal antibody against human TLR2, TLR4 (1:100, Santa Cruz Biotechnology, Inc., Delaware, CA, USA) and Fas (1:100, Neomarkers, Inc., Fremont, CA, USA). After rinsing with PBS, they were overlaid with biotinylated horse anti-IgG (R.T.U. Vectastatin® Universal ABC Elite kit) at room temperature for 1 hour. After rinsing with PBS, they were reacted with avidin-biotin-peroxidase complex solution at room temperature for 1 hour. Visualization of the enzyme reaction was performed with the Vector® NovaRED™ substrate kit (Vector Laboratories, Inc., Burlingame, CA, USA). After the chromogen reactions, the sections were rinsed and then counterstained with Mayer's hematoxylin. After rinsing, the sections were then dehydrated and covered with permount (Fisher Scientific, Fair Lawn, NJ, USA). They were examined by light microscopy to assess the histological changes.
Specimens for the human sebocyte culture were obtained, with the consents of the patients, from the non-balding occipital scalp region of patients with androgenic alopecia, during hair transplantation at Kyungpook National University Hospital (Daegu, South Korea). Defined Keratinocyte-SFM and its growth supplement (DMEM; Gibco BRL, Grand Island, NY, USA), supplemented with Antibiotic-Antimycotic (10µl/ml) were used as a culture medium. Primary cultures of sebocytes were performed according to the method described previously9. Briefly, sebaceous glands were isolated from dissected hair follicles under a binocular microscope, and transferred to a tissue culture dish. The cells were maintained in DMEM at 37℃ in a humidified atmosphere of 5% CO2. The explants were left for 5 days, and the medium, Defined Keratinocyte-SFM, was changed every other day.
After the sebocytes had grown to confluence, the medium was removed and a serum-free medium containing 1 mM ALA (Sigma, St Louis, MO, USA), was added. The cells were allowed to take up ALA for 24 hours. The medium containing ALA was then removed and irradiation was immediately performed, using an incoherent light source with a narrow band LED (Omnilux®; 633±3 nm), with a light intensity of 105 mW/cm2 and light doses of 1~15 J/cm2 at 10~15 cm over the plate.
There were four treatment groups: one group of sebocytes received both ALA and LED (PDT group), the others were controls. The first one received neither sensitizer nor irradiation, the second was irradiated only with LED, and the last was treated with ALA only. Apoptotic response of the sebocytes was shown with terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining.
DNA strand breaks which are associated with the apoptotic response can be identified by labeling, in an enzymatic reaction, the free 3'- OH termini with modified nucleotides. Based on this principle of TUNEL assay, apoptosis in acne lesions was examined using in situ cell death detection kit (Roche Diagnostics GmbH, Penzberg, Germany). The sections were fixed with 4% PFA and 3% H2O2 in methanol. They were then treated with proteinase K working solution for 30 minutes, at 37℃. The sections were treated with TUNEL reaction mixture, composed of TdT, and fluorescein labels which were incorporated in nucleotide polymers were detected and quantified by fluorescence microscopy.
Statistical analysis was carried out using the paired t-test to compare the changes of acne lesion counts and SO from baseline. Also, correlation coefficient test was done to evaluate the correlation between the intensity of light for PDT and apoptotic cell counts. Statistical significance was defined as a p-value of less than 0.05.
Of the 12 patients, 10 completed the three sessions of PDT and 8 patients (7 females and 1 male) were followed up for 3 months after final PDT session; the remaining three did not return to the clinic for these appointments. Ten sets of biopsy specimens were prepared. The average age of the 10 patients who completed the three session of PDT was 28 years (range 19 to 38 years) and the duration of suffering of facial acne varied from 3 months to 25 years. Their Fitzpatrick's skin phototype was IV or V. One patient was a hepatitis B virus carrier without symptoms and the others had no underlying disease. Table 1 shows the demographic characteristics of patients.
Fig. 1 shows that there was considerable overall improvement of facial acne. There was a significant reduction in the number of inflammatory acne lesion after the PDT compared to baseline (Fig. 2A); the mean reduction of the inflammatory acne lesion count was 52% at 4 weeks after the final session of PDT (p<0.05). Suppression of acne was maintained relatively well for 8 weeks after the final PDT (p<0.05). The non-inflammatory lesion count showed a gradual improvement from baseline to 8 weeks after the final PDT (Fig. 2B), but was not statistically significant.
Sebum excretion significantly decreased 2 weeks after the first PDT session (mean 44%) except in one patient, whose baseline SO level was low (p<0.05). Sebum excretion slowly returned to an average 89% of baseline level, 8 weeks after the termination of PDT (Fig. 2C).
All patients experienced a mild stinging sensation and itching. All patients showed mild to moderate erythema and edema which lasted for two to three days after each treatment. Erythema was most prominent the day after each PDT. One patient showed a sunburn-like erythema and edema after the first PDT, which was well tolerated and healed without any sequelae within 2 weeks. Some patients experienced reactive acne eruption with tiny pustules for a few days after PDT. This improved in a week.
Post-inflammatory hyperpigmentation was seen in two patients who showed relatively severe erythema, lasting for 2 or 3 months. One patient developed melasma after the PDT which was not recognized before the start of therapy.
Apoptotic cell death before and after PDT was shown by TUNEL assay (Fig. 3). Only faint apoptotic cells were detected in the basal layer of the sebaceous glands before PDT (Fig. 3a, b), however we observed many apoptotic cells in the sebaceous glands after the PDT (Fig. 3c, d). The majority of apoptotic cell death was seen in the center of the sebaceous glands. We found this expression pattern in 8 of our 10 cases (80%).
Apoptosis can occur via the Fas/FasL pathway. Immunoreactivity of Fas was increased in the sebaceous glands of the samples after PDT compared to baseline (Fig. 4). Relatively clear contrast was observed in 5 out of 7 cases (71%).
We revealed the expression of TLR-2 and TLR-4, which trigger inflammatory cytokine responses by P. acnes, after PDT5. In immunohistochemical staining of TLR-2 at baseline (before PDT), we found intense immunoreactivity in 5 cases out of 10, in the suprabasal layer of the epidermis, in the sebaceous gland, and in the follicular epithelium (Fig. 5a, b). In these cases, the expression of TLR-2 decreased in the epidermis and sebaceous glands after PDT therapy compared to baseline (Fig. 5c, d). In others, however, TLR-2 expression was not increased at baseline and there was also no noticeable change associated with treatment. With respect to TLR-4, three out of ten cases showed a similar pattern of decreasing expression after PDT compared to baseline (Fig. 6). Overall, the immunoreactivity pattern of TLR-4 was similar to TLR-2.
The semiquantitative analysis of staining results for TUNEL, Fas, and TLRs was expressed in Table 2.
To confirm the apoptosis of sebocytes after the PDT in vitro, we performed TUNEL assay using the cultured sebocytes obtained from the human scalp. We observed increased apoptotic cells after ALA-PDT by TUNEL stain. TUNEL positive cell count was 687, 452, 501 and 661 per 1.8 cm2 sized well on average, when cultured sebocytes were treated with PDT in light dose of 1, 2, 5, and 10 J/cm2, respectively. On the other hand, TUNEL positive cell count was 266 in groups of ALA only and 205 in groups of LED only. Apoptotic cells of ALA-PDT group were two-and-a-half or more times in number than groups with only ALA or only LED (Fig. 7). However, there was no significant difference in the number of apoptotic cells between the 1, 2, 5, and 10 J/cm2 PDT group. We used 1 mM ALA concentration because there was no difference in effect between 1 mM, 1.5 mM, and 2 mM ALA concentration. Cell viability was not significantly reduced by 1 mM ALA uptake.
Our clinical assessment showed a significant reduction in the number of inflammatory acne lesions after three sessions of PDT. There was a decrease of fifty three percent of the inflammatory acne lesions, which was maintained for the 8 weeks following the 3 PDT sessions. Additionally, the number of non-inflammatory acne was also decreased, but a direct correlation with PDT was not found.
The most recent studies on acne with PDT reported that a better effect was seen on the inflammatory acne lesion than the non-inflammatory type. This implies that PDT may work through modulation of inflammation. We hypothesized that improvement of inflammatory acne by ALA-PDT might be related with TLRs expression. In this study, decreased expression of TLR-2 and TLR-4 in the sebaceous glands and epidermis after ALA-PDT was observed in some acne patients whose TLR expression was increased at baseline.
TLRs are transmembrane signaling receptors of the innate immune system, activated by pathogen exposure10,11. TLR-2 and TLR-4 are relatively well known receptors that are expressed on human keratinocytes11. TLR-2 defends against Gram-positive bacteria, Mycobacteria, Mycoplasma, and fungi11. TLR-4 mainly binds to the LPS of Gram-negative bacteria11. Jugeau et al.12 demonstrated that P. acnes induces TLR-2 and TLR-4 expression in acne lesion; this could be related with acne-linked inflammation. Likewise, activated TLR-2 in acne triggers inflammatory cytokine responses such as IL-8, IL-12 and so on13. Decreased TLR-2 or TLR-4 expression after PDT may be related to improvement of acne after PDT through a reduction of inflammation caused by P. acnes. The most typical drug for acne, synthetic retinoid, is known to exert an anti-inflammatory activity. Lately, all-trans retinoic acid and adapalene were reported to down-regulate TLR-2 expression in an in vitro study14,15. These data suggest that TLRs might provide a novel target for acne vulgaris by modulating the immune responses.
In this study, not all perilesional sebaceous glands and epidermis of acne patients showed increased expression of TLR-2 and TLR-4 at baseline. Constitutive expression of TLR-2 and TLR-4 on the human epidermis has been reported in previous studies16,17. It is presumed that there is individual difference of TLRs expression among acne patients, according to the degree of inflammation.
P. acnes produces porphyrins, but in amounts too small to evoke the adequate photodynamic effect18. With ALA, the increase of porphyrin production enhances the photodynamic effect. Bacterial (P. acnes) killing or inactivation is one of the suggested mechanisms, but phototoxic damage of the sebaceous glands is another possibility18. Pp IX is accumulated in the sebaceous glands because of its strong lipophilicity after systemic or topical application7,19. Accordingly, the target of PDT on acne may be the sebaceous gland.
Reduced sebum secretion after ALA-PDT suggests damage of the sebaceous glands. Vacuolization and degeneration of sebaceous glands were found after ALA-PDT for treatment of acne20,21. Itoh et al.21 inferred tissue-damage mechanism is apoptosis-like oxidative damage. PDT has been reported to treat tumors by inducing apoptosis22. Webber et al.23 reported the apoptotic response of ALA-induced photo-damage in colon cancer, however it is not well defined whether the apoptotic response of sebaceous glands would occur in PDT on acne.
This study demonstrated that apoptotic photo-damage of sebaceous glands is one mechanism of ALA-PDT efficacy on acne. We showed this in both our in vitro and in vivo studies. From the in vivo study, we found many apoptotic cells in the sebaceous gland after ALA-PDT, which were rarely detected before PDT. Also, from the in vitro study, the number of apoptotic sebocytes increased after ALA-PDT was higher than the ALA only group or the LED only group. Bednarz et al.22 reported that Pp IX, which is a metabolite of ALA, could induce apoptosis in HeLa cells without light. Considering the unavoidable light during the experiment, ALA itself may evoke apoptosis of sebocytes after changing into Pp IX. Positive Fas reactivity suggests that an apoptotic response occurs via the Fas/FasL pathway. However, other pathways of apoptosis, such as the caspase cascade, should be also evaluated in the future.
In our study, there was marked decrease in SO after the first session, but which was not maintained afterwards. The improvement of inflammatory acne lesion was relatively well maintained, even though sebum excretion returned to the baseline 8 weeks after PDT. Most of the other clinical studies revealed decreased SO after PDT, but a correlation between SO and PDT remains controversial. Hongcharu et al.20 noted a significant decrease in SO after ALA-PDT and showed the atrophic sebaceous glands at even 20 weeks following the four treatments. On the other hand, similar to our results, Pollock et al.24 demonstrated a significant reduction of inflammatory acne lesion counts with no significant reduction of sebum excretion. Further studies are required to detect the change of sebum secretion or sebaceous glands by PDT.
From our results we see that photodamage of sebaceous glands does not on its own explain the effect of PDT on acne. As previously mentioned, some clinical reports, including our data, noted that a correlation was not found between the SO and improvement of acne24. The SO recovery suggests the regeneration of once damaged sebaceous glands through PDT. So, we inferred that photo-damage by apoptosis of sebaceous glands may contribute to improvement of acne in early stages, and immunomodulation of inflammation by TLR-2 or TLR-4 can maintain the improvement of inflamed acne into late stages.
The apoptotic response did not differ between the ALA concentrations in this study. Kosaka et al.19 showed that the induction of Pp IX was dependent on the amount of ALA applied. There may, though, be a saturation dose of ALA. We could not find this concentration, but there was little difference between 0.2, 1, 1.5, and 2 mM ALA. We expected greater apoptotic response in proportion to light intensity, however from the in vitro study, there was no statistical difference in the number of apoptotic cells between the 1, 2, 5, 10 J/cm2 group. One clinical report noted that no difference was found between the high and low light doses in acne patients and so the lowest light dose should be used in order to minimize side effect19. From this point of view, it seems that there is no advantage of raising the light intensity to enhance the PDT effect, as long as the intensity of light is above a certain level. However, because real sebaceous glands are located about 1~2 mm below the epidermal surface20, light intensity below the minimum may not cause an adequate photodynamic effect under actual PDT.
No serious side effect or treatment resistance was found in this study. Our patients complained of mild stinging or transient erythema, and the consensus reply was that avoidance of sun exposure for 2 days was most inconvenient. Rapidly disintegrated chemicals may be a better choice, in heeding to these concerns.
Transient post-inflammatory hyperpigmentation, or the instigation of the appearance of concealed melasma was the only concerning side effect noted among the study subjects. These pigmentation problems are much more frequent in Asians than Caucasians. Post-inflammatory hyperpigmentation makes acne patients (especially young female patients) lose their compliance. The patient who developed melasma after PDT had to be given whitening treatment after this study. Careful selection of patient and proper PDT light adjustment are required to achieve good effect with few/low side effects.
Our non-comparative trial evaluated the efficacy of ALA-PDT in acne treatment as well as clarified its mechanism of action. The limitation of our study is its small size, but this is the first study to show apoptosis of sebocytes and sebaceous glands after PDT through in vivo as well as in vitro testing.
In conclusion, our data suggest that topical ALA-PDT for acne can induce apoptosis of sebocytes and down-regulate their TLR-2 and TLR-4 expression, thereby ameliorating acne vulgaris.
Figures and Tables
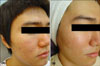 | Fig. 1Photography of patient 6. (A) Before the treatment. (B) Five weeks after the final treatment, acne lesions were cleared. |
 | Fig. 2The changes of mean inflammatory acne lesion count (A), non-inflammatory lesion count (B) and sebum output (C) were shown. Photodynamic therapy was performed at week 0, 2 and 4. |
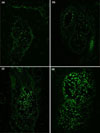 | Fig. 3Apoptotic sebocytes before (a, b) and after (c, d) photodynamic therapy, by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) staining (×400). (a, c) Positive cells are increased in the sebaceous glands of patient 1. (b, d) Similar patterns are seen in patient 7. |
 | Fig. 4The change of Fas expression before (a) and after photodynamic therapy (PDT) (b). Increased Fas expression is detected in the sebaceous gland after PDT (×40, inlet: ×400). |
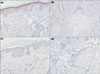 | Fig. 5Immunohistochemical staining of toll-like receptor (TLR)-2 in patient 3 (×400). (a, b) TLR-2 is found in the epidermis and sebaceous glands of the peri-lesional areas. It is strongly positive especially in the granular layer and follicular epithelium of sebaceous gland. (c, d) A marked decrease in TLR-2 in the peri-lesional areas after photodynamic therapy. |
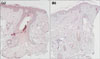 | Fig. 6Immunohistochemical staining of toll-like receptor (TLR)-4 in patient 6 (×100). (a) Similar staining pattern to TLR-2 is observed in TLR-4 staining. (b) Decreased staining of TLR-4 was seen after photodynamic therapy. |
 | Fig. 7Increased apoptotic cells are observed by TUNEL assay after photodynamic therapy (PDT) compared with control (×40). No difference between various light intensities. (a) No treatment, (b) light treatment only (5 J), (c) aminolevulinic acid incubation only, (d) PDT with 1 J/cm2, (e) PDT with 2 J/cm2, (f) PDT with 5 J/cm2, (g) PDT with 10 J/cm2. |
References
1. White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998. 39:S34–S37.

2. Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997. 136:66–70.

3. Webster GF. Acne vulgaris. BMJ. 2002. 325:475–479.
5. Leyden JJ, McGinley KJ, Cavalieri S, Webster GF, Mills OH, Kligman AM. Propionibacterium acnes resistance to antibiotics in acne patients. J Am Acad Dermatol. 1983. 8:41–45.
6. Zeitouni NC, Oseroff AR, Shieh S. Photodynamic therapy for nonmelanoma skin cancers. Current review and update. Mol Immunol. 2003. 39:1133–1136.

7. Divaris DX, Kennedy JC, Pottier RH. Phototoxic damage to sebaceous glands and hair follicles of mice after systemic administration of 5-aminolevulinic acid correlates with localized protoporphyrin IX fluorescence. Am J Pathol. 1990. 136:891–897.
8. Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992. 14:275–292.
9. Fujie T, Shikiji T, Uchida N, Urano Y, Nagae H, Arase S. Culture of cells derived from the human sebaceous gland under serum-free conditions without a biological feeder layer or specific matrices. Arch Dermatol Res. 1996. 288:703–708.

11. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000. 406:782–787.

12. Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005. 153:1105–1113.

13. Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002. 169:1535–1541.

14. Liu PT, Krutzik SR, Kim J, Modlin RL. Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005. 174:2467–2470.

15. Tenaud I, Khammari A, Dreno B. In vitro modulation of TLR-2, CD1d and IL-10 by adapalene on normal human skin and acne inflammatory lesions. Exp Dermatol. 2007. 16:500–506.

16. Ku JK, Kwon HJ, Kim MY, Kang H, Song PI, Armstrong CA, et al. Expression of Toll-like receptors in verruca and molluscum contagiosum. J Korean Med Sci. 2008. 23:307–314.

17. Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003. 148:670–679.

18. Ashkenazi H, Malik Z, Harth Y, Nitzan Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol Med Microbiol. 2003. 35:17–24.

19. Kosaka S, Kawana S, Zouboulis CC, Hasan T, Ortel B. Targeting of sebocytes by aminolevulinic acid-dependent photosensitization. Photochem Photobiol. 2006. 82:453–457.

20. Hongcharu W, Taylor CR, Chang Y, Aghassi D, Suthamjariya K, Anderson RR. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000. 115:183–192.

21. Itoh Y, Ninomiya Y, Tajima S, Ishibashi A. Photodynamic therapy of acne vulgaris with topical delta-aminolaevulinic acid and incoherent light in Japanese patients. Br J Dermatol. 2001. 144:575–579.

22. Bednarz N, Zawacka-Pankau J, Kowalska A. Protoporphyrin IX induces apoptosis in HeLa cells prior to photodynamic treatment. Pharmacol Rep. 2007. 59:474–479.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download