Abstract
Lichen sclerosus et atrophicus (LSA) is an inflammatory disease that primarily causes anogenital lesion in middle aged women. We present here a case of facial LSA with an asymptomatic, well-demarcated, whitish to bluish, atrophic patch in a linear pattern on the forehead of a 48-year-old woman. This case showed an atypical clinical presentation and it mimicked en coup de sabre, but the histopathologic results confirmed the diagnosis of LSA.
Lichen sclerosus et atrophicus (LSA) is a rare chronic inflammatory dermatosis with anogenital and extragenital presentations. Women in the fifth or sixth decade of life and children younger than the age of 10 years are usually affected by this disease. The anogenital lesions can cause severe discomfort (dyspareunia, dysuria, pruritus and painful defecation) and manifest with erosions, porcelain-white plaques, papules and wide degrees of sclerosis. Potent topical corticosteroids associated with skin care are thought to be the most successful therapy and calcineurin inhibitors are also effective according to recent reports.
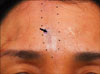
A 48-year-old Korean woman presented with a 1-year history of a linear atrophic patch on the forehead and this had gradually spread. The patient had no significant past illness. Physical examination revealed an well-demarcated, whitish to bluish, atrophic patch in a linear pattern on the forehead (Fig. 1). There was a focal hyperpigmented area within the lesion. The patient had cosmetic concerns, but otherwise she had no symptoms or evidence of mucosal or other cutaneous involvement. Histological examination of a biopsy taken from the center of the lesion showed hyperkeratosis, thinning of the epidermis, loss of the rete ridges, focal basal cell vacuolization, pigmentary incontinence, edema and hyalination of the papillary dermis along with a moderate lymphomononuclear cell infiltrate (Fig. 2A). Special staining for elastic fiber showed scanty elastic tissue in the dermis (Fig. 2B). Based on the clinical and histological findings, this case was diagnosed as linear LSA along the Blaschko's line of the face. Partial improvement was noted with topical steroid ointment and steroid intralesional injection at the 3-month follow up. Close patient follow-up is planned because there have been reports of transition of LSA to localized scleroderma on sequential biopsies.
LSA is an inflammatory dermatosis of an unclear pathogenesis and it primarily affects the vulvar, perineal and perianal skin of prepubertal, perimenopausal and postmenopausal women.
However, extragenital LSA is not uncommon and it was found in 805 of 5,207 cases reviewed by Meffert et al.1 and it was most common on the neck, shoulders and upper portion of the trunk. It is generally asymptomatic, but it can occasionally be pruritic. Less common sites include the palms, soles, scalp and face1.
The first case report of a linear LSA was described in 1995 by Izumi and Tajima2. Thereafter, a handful of cases of linear LSA have been reported, among which some developed in a pattern corresponding to the lines of Blaschko. Kim and Lee3 has summarized 6 cases of linear LSA along the Blaschko's lines, and this occurred on the trunk, limbs or face. Out of the 3 reported cases of linear LSA that appeared on the face, one case showed facial lesion following the Blaschko's line without any oral mucosal lesion, and the other 2 cases showed additional oral mucosal involvement3-5. This is the second case of linear LSA without oral mucosal lesion and the lesion presented on the face following the line of Blaschko6.
The clinical, histological differential diagnosis between LSA and morphea is essential for dermatologists. In our case, the clinical differential diagnosis included linear scleroderma or en coup de sabre since its predilection site was involved. However, microscopic examination disclosed the characteristic features of LSA. The relationship between LSA and morphea is still controversial. However, several histopathological findings such as follicular plugging, epidermal thinning with hydropic basal degeneration, thick lichenoid lymphocytic infiltration and decreased elastic fibers in the upper dermis favor the diagnosis of LSA7,8. So, the histopathological findings are crucial for making the differential diagnosis between LSA and morphea.
Many authors have described coexistent LSA and morphea9,10. Morphea and LSA coexisting in the same biopsy specimen has also been described10. With sequential biopsies, several investigators have reported transition from LSA to morphea or vice versa10,11. However, other investigators believe there are enough clinical and histologic differences between LSA and morphea to argue that they are distict diseases and that coexistent lesions are coincidental1. It is possible that this LSA case could progress to en coup de Sabre, and this case may be a transitional form of linear scleroderma en coup de Sabre5. Curing LSA is almost impossible, but treatment can relieve the subjective symptoms and prevent further progression. Various therapeutic options such as topical corticosteroids, retinoids, phototherapy12, estrogen, vitamins, topical tacrolimus13 and surgical removal have shown only limited responses. In our case, partial improvement was noted with topical steroid ointment and steroid intralesional injection at the 3-month follow up. According to the previous reports, transition of LSA to localized scleroderma in sequential biopsies is possible, so we will monitor this patient to detect any progression or change of the linear facial lesion.
Considering the possibility of progression of linear LSA to en coup de Sabre, early detection and appropriate treatments are necessary for such a patient.
Figures and Tables
Fig. 2
(A) Thinning of the epidermis, loss of the rete ridges, focal basal cell vacuolization, pigmentary incontinence, edema and hyalination of the papillary dermis along with a moderate lymphomononuclear cell infiltrate are noted (H&E stain, ×100). (B) Special staining for elastic fiber showed scanty elastic tissue in the dermis (Elastic fiber, ×100).

References
2. Izumi T, Tajima S. A case of linear type of lichen sclerosus et atrophicus? J Dermatol. 1995. 22:279–282.

3. Kim YJ, Lee ES. Case of sequentially occurring lesions of facial lichen sclerosus following the lines of Blaschko. J Dermatol. 2007. 34:201–204.

4. Kaur S, Thami GP, Kanwar AJ, Mohan H. Linear oro-facial lichen sclerosus. Clin Exp Dermatol. 2002. 27:467–470.

5. Walsh SN, Jorizzo JL, Haverstock C, Sangüeza OP. A linear orofacial macule. Am J Dermatopathol. 2008. 30:194–195.

6. Happle R, Assim A. The lines of Blaschko on the head and neck. J Am Acad Dermatol. 2001. 44:612–615.

7. Hengge UR. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Lichen sclerosus. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;546–550.
8. McNiff JM, Glusac EJ, Lazova RZ, Carroll CB. Morphea limited to the superficial reticular dermis: an underrecognized histologic phenomenon. Am J Dermatopathol. 1999. 21:315–319.

9. Tremaine R, Adam JE, Orizaga M. Morphea coexisting with lichen sclerosus et atrophicus. Int J Dermatol. 1990. 29:486–489.

10. Sawamura D, Yaguchi T, Hashimoto I, Nomura K, Konta R, Umeki K. Coexistence of generalized morphea with hisotological changes in lichen sclerosus et atrophicus and lichen planus. J Dermatol. 1998. 25:409–411.

11. Nishioka S. Histological comparison of morphea and lichen sclerosus et atrophicus. Kurume Med J. 1997. 44:83–90.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download