Abstract
Background
Many variants of dermatofibromas have been described, and being aware of the variants of dermatofibromas is important to avoid misdiagnosis.
Objective
We wanted to evaluate the clinical and pathologic characteristics of 122 cases of dermatofibromas.
Methods
We retrospectively reviewed the medical records and 122 biopsy specimens of 92 patients who were diagnosed with dermatofibroma in the Department of Dermatology at Eulji Hospital of Eulji University between January 2000 and March 2010.
Results
Nearly 80% of the cases occurred between the ages of 20 and 49 years, with an overall predominance of females. Over 70% of the lesions were found on the extremities. The most common histologic variant was a fibrocollagenous dermatofibroma (40.1%). Other variants included histiocytic (13.1%), cellular (11.5%), aneurysmal (7.4%), angiomatous (6.5%), sclerotic (6.5%), monster (4.9%), palisading (1.6%) and keloidal dermatofibromas (0.8%). There were 9 dermatofibromas (7.3%) that were the mixed type with two co-dominant histologic features.
Dermatofibroma (benign fibrous histiocytoma) is a common skin lesion and it accounts for approximately 3% of the skin lesion specimens received by one dermatopathology laboratory1. There is a predilection for dermatofibroma to develop on the extremities, and particularly on the lower extremities of young adults. There is a female preponderance amongst the patients with dermatofibroma1. Dermatofibroma is round or ovoid, firm, dermal nodules and they are usually <1 cm in diameter2. The diagnosis is usually straightforward if the classical clinical and pathologic features are evident. However, many variants of dermatofibromas have been described, and knowledge of these variations is important to avoid a misdiagnosis of a possibly more aggressive tumor. The main histologic variants include fibrocollagenous, cellular3, histiocytic4, lipidized5, angiomatous6, aneurysmal7, clear cell8, monster cell9, myxoid10, keloidal11, palisading12, osteoclastic13 and epithelioid dermatofibroma14. There are no prior Korean reports regarding the detailed classification of the histologic features of dermatofibromas. Therefore, we report here on a series of 122 cases of dermatofibroma and we discuss the clinical and histopathologic features.
We performed a retrospective review of the medical records and 122 biopsy specimens of 92 patients who were diagnosed with dermatofibroma in the Department of Dermatology at Eulji Hospital of Eulji University between January 2000 and March 2010.
The following clinical data was collected: age, gender, duration, size, number, the distribution of the lesions, symptoms, color, a history of trauma, the clinical configuration and the clinical diagnosis of the lesions.
All of the cutaneous samples were fixed in formalin, processed and embedded in paraffin. The sections were stained with hematoxylin and eosin. The samples were classified as variants of dermatofibromas (fibrocollagenous, sclerotic, cellular, histiocytic, aneurysmal, angiomatous, keloidal, monster, palisading and mixed) according to the dominant pathologic features. When the cellular type of dermatofibroma was suspected, immunohistochemical staining (CD34 and factor XIIIa) was performed to differentiate it from a dermatofibrosarcoma protuberans (DFSP). In addition, the depth of invasion, the epidermal changes (hyperkeratosis, acanthosis and basal layer hyperpigmentation), the presence of a grenz zone, formation of lymphoid nodules and mucin deposition were determined.
Among the 92 studied patients, dermatofibroma was more common in females than in males (26 and 68, respectively; the male-to-female ratio was 1:2.6). The youngest patient was 8 years of age and the oldest patient was 72 years of age, and the mean age was 36.2 years. Greater than 80% of the tumors were diagnosed in patients between 20 and 49 years of age. The highest frequency of tumor according to age was between 20 and 29 years of age (31.5%; Fig. 1).
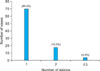
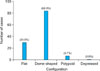
The number of tumors that persisted for <2 years was 71 (58.2%) and 51 tumors (41.8%) persisted for >2 years. The size of the tumor on examination varied from 0.3~5 cm, with most of the tumors <2 cm in size (n=117, 85.2%; Fig. 2). The tumors appeared as various colored lesions. The most common color was brown (n=51, 41.8%). The second most common color was pink-red (n=40, 32.8%), followed by skin color (n=28, 23.0%). Three cases (2.45%) were blue in color. The majority of the patients had a single lesion (n=74, 80.4%; Fig. 3). Based on the clinical configuration of the lesion, the tumors were divided into the following four groups: flat, dome-shaped, polypoid and depressed. The most common configuration was dome-shaped (n=84, 68.9%; Fig. 4).
The most common location of tumors was the legs (n=52, 42.6%), followed by the arms (n=37, 30.3%). Table 1 lists the distribution of the dermatofibromas. The arms, legs, buttocks, chest, face, abdomen, back, hand, and feet were affected in decreasing frequency. No dermatofibroma was observed on the scalp or neck (Fig. 5).
Three tumors occurred at the site of an insect bite, and one case occurred at the site of a piercing. Most of the tumors were asymptomatic (n=71, 58.2%). Twenty-five (20.5%) tumors were tender, 14 were painful (11.5%) and 12 tumors were pruritic (58.2%).
The correct pre-operative diagnosis was made in 100 cases (82.2%). The most common clinical impression was a dermatofibroma with a wide range of other possible diagnosis, including an epidermal cyst (8.7%), pilomatricoma (3.5%), eccrine poroma (2.9%), dermatofibrosarcoma protuberans (2.9%), intradermal nevus (2.9%), leiomyoma (2.9%), keloid (2.9%), soft fibroma (1.5%) and trichilemmal cyst (1.5%).
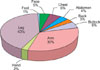
The most common variant was a fibrocollagenous dermatofibroma (n=49, 40.1%). The other variants included histiocytic (n=16, 13.1%), cellular (n=14, 11.5%), aneurysmal (n=9, 7.4%), angiomatous (n=8, 6.5%), sclerotic (n=8, 6.5%), monster (n=6, 4.9%), palisading (n=2, 1.6%) and keloidal dermatofibroma (n=1, 0.8%). There were 9 mixed-type dermatofibromas (7.3%) with 2 codominant histologic features (Fig. 6 and 7).
The triad of epidermal changes included hyperkeratosis, acanthosis and basal layer hyperpigmentation. Hyperkeratosis, acanthosis and basal layer pigmentation were noted in 67.2%, 75.4% and 72.1% of the samples, respectively, and a grenz zone was noted in 71.3% of the samples. For the aneurysmal-type dermatofibroma, acanthosis was observed at a lower rate than that for the other subtypes of dermatofibromas (33.3%; p<0.05). Basal pigmentation was observed at a lower rate in the cellular-type dermatofibromas (42.9%; p<0.05). There was no specific subtype that was differed from the other subtypes according to the presence of grenz zone formation (p>0.05). The depth of invasion of the tumor was the dermis, superficial subcutis and deep subcutis in 57.4%, 39.3% and 3.3% of the tumors, respectively. Greater than one-half of the cases of the cellular (50%), aneurysmal (77.8%) and monster types of dermatofibroma (66.7%) had invaded the subcutaneous tissues. However, only the aneurysmal type of dermatofibroma had statistical significance with respect to a deep depth of invasion to the subcutaneous tissues (p<0.05). The presence of lymphoid nodules in the dermo-subcutaneous junction was observed in 9.8% of the tumors, and mucin deposition was noted in 18.9% of the tumors. Lymphoid nodules were observed in 16.3% of the fibrocollagenous types of dermatofibroma, which was a higher frequency compared to that of the other subtypes of dermatofibroma (p<0.05). The cellular type of dermatofibroma had the highest frequency of mucin deposition (28.6%; p>0.05), yet there was no specific subtype that was differed from the other subtypes according to mucin deposition (p>0.05; Table 1; Fig. 8).
Dermatofibroma is one of the most common types of benign, cutaneous, soft tissue tumors. Dermatofibroma is most often found in the middle-aged adults and it has a slight female predominance. The majority of lesions are located on the limbs or the trunk, and they present as small, raised, hyperkeratotic, cutaneous nodules <1 cm in diameter with a red-brown surface. A significant proportion of cases are associated with minor local trauma, and especially insect bites15. The lesions are most often solitary, but 2~5 lesions are present in approximately 10% of individuals4. In the current study, most of the clinical characteristic features were consistent with those of the previous reports16,17.
The results showed that most tumors presented at between 20 and 49 years of age with an overall predominance of females. Over 70% of the cases occurred on the extremities, and they appeared dusky brown in color with a smooth surface. However, unlike the previous reports, the most common diameter in our study was 1~2 cm (48.4%). Indeed, prior Korean studies have reported the most common diameter to be 1~5 mm16,17. Only 3 cases of the tumor occurred at sites of insect bites and most of the tumors were asymptomatic (58.2%). In addition, more than 2 lesions were present in 14.7% of the patients.
Histopathologically, dermatofibroma is composed of a variable mixture of fibroblast-like cells, histiocytes (some of which may be xanthomatous or multinucleate) and blood vessels. The term 'nodular subepidermal fibrosis,' 'histiocytoma' and 'sclerosing hemangioma' have been used in the past for variants in which one of the three components predominated. The lesions are currently referred to as the fibrocollagenous, histiocytic, and aneurysmal variants to reflect the difference in composition18. In addition, numerous other variants have recently been described. The 122 cases of dermatofibromas were classified into variants according to the dominant pathologic features. The most common variant was a fibrocollagenous dermatofibroma (40.1%). The other variants included histiocytic (13.1%), cellular (11.5%), aneurysmal (7.4%), angiomatous (6.5%), sclerotic (6.5%), monster (4.9%), palisading (1.6%) and keloidal dermatofibroma (0.8%), in decreasing frequency. In addition, the tumors that occurred simultaneously in one patient tended to have similar histologic features.
The fibrocollagenous type of dermatofibroma has a predominance of collagen and fibroblast-like cells in an irregular or whorled arrangement18. One variant of the fibrocollagenous type, the 'palisading dermatofibroma,' has nuclear palisading and prominent verocay-like bodies in part of the lesion12. The cases with a prominent storiform pattern are referred to as cellular dermatofibroma3. The histiocytic variant has nests and sheets of histiocytes in a poorly cellular collagenous stroma. There are often many foam cells and giant cells, which may be the foreign body or touton type. Hemosiderin and lipid are commonly present4. In the angiomatous (vascular variant) type, there are numerous small branching vessels in a variable collagenous stroma6. The aneurysmal variant is distinct, with blood-filled spaces occupying up to one-half of the lesion. The vascular channels are surrounded by histiocytes that contain hemosiderin, foam cells and fibroblasts7. In addition, large, bizarre cells with abundant foamy cytoplasm and hyperchromatic nuclei have been reported in some dermatofibromas. These 'monster cells' can be binucleated or multinucleated9. Two other rare variants are the keloidal dermatofibroma with areas of thick eosinophilic collagen, and the sclerotic dermatofibroma with areas of sclerosis11,19.
Dermatofibroma is a poorly demarcated tumor centered on the dermis. There is sometimes extension into the superficial subcutis, which may take the form of a septal extension or a well-demarcated bulge20. A pure subcutaneous form is exceedingly rare21. The results of the current study showed that 39.3% of the cases invade the subcutis and 3.3% of the cases invade the deep subcutis. Greater than one-half of the cases of the cellular (50%), aneurysmal (77.8%) and monster types (66.7%) had invaded the subcutaneous tissue. In addition, the aneurysmal type was statistically significant with respect to the depth of invasion (p<0.05). There were no cases with the pure subcutaneous form of a dermatofibroma.
Dermatofibromas, including the variants, may be associated with acanthosis or hyperplasia of the overlying epidermis and hyperpigmentation of the basal layer22. It has been suggested that epidermal growth factor may play a role in the pathogenesis of the epidermal hyperplasia23. In the current study, the aneurysmal type of dermatofibroma had a lower frequency of acanthosis (33.3%) and the cellular type of dermatofibroma had a lower frequency of basal pigmentation (42.9%; p<0.05). However, there were no specific subtype differences from the other subtypes according to the presence of the grenz zone. Other histologic changes observed with dermatofibroma include the presence of lymphoid nodules and mucin deposition10,24. The presence of lymphoid nodules at the dermo-subcutaneous junction was observed in 9.8% of the cases in our study, and mucin deposition was noted in 18.9% of the cases. Lymphoid nodules were observed in 16.3% of the cases of the fibrocollagenous type of dermatofibroma (p<0.05).
The results of the current study are consistent with the previous reports on the clinical features of dermatofibroma. However, we observed several characteristic subtypes of dermatofibromas and we compared the frequency of the histologic subtypes. We assume the reason why most of the statistical results were nonsignificant was the small sample size in our study. Consequently, we suggest that a larger study or a multicenter-based study is needed to identify more subtypes, such as osteoclastic and epithelioid dermatofibroma, and to more accurately analyze the correlation between the subtypes and clinical/histologic features.
Figures and Tables
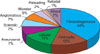 | Fig. 6Classification into the variants of dermatofibroma. The most common histologic variant was a fibrocollagenous dermatofibroma (n=49, 40.1%), followed by histiocytic (n=16, 13.1%), cellular (n=14, 11.5%), aneurysmal (n=9, 7.4%), angiomatous (n=8, 6.5%), sclerotic (n=8, 6.5%), monster (n=6, 4.9%), palisading (n=2, 1.6%) and keloidal dermatofibroma (n=1, 0.8%). |
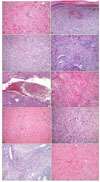 | Fig. 7Various histologic types of dermatofibroma. (A) The fibrocollagenous type shows a predominance of collagen and fibroblasts in a whorled arrangement (H&E, ×100). (B) The histiocytic type shows sheets of histiocytes with many melanophages (H&E, ×100). (C) Foamy histiocytes in the histiocytic type (H&E, ×40). (D) The cellular type showing high cellularity with short fascicular and storiform growth (H&E, ×100). (E) The aneurysmal type is characterized by blood-filled spaces reminiscent of cavernous hemangioma (H&E, ×40). (F) The sclerotic type with marked hyalinized eosinophilic collagen bundles (H&E, ×100). (G) The angiomatous type with numerous small branching vessels in a collagenous stroma (H&E, ×100). (H) The monster type with multinucleate giant cells with large, hyperchromatic, bizarre nuclei (H&E, ×100). (I) The palisading type with verocay-like body in the center of the lesion (H&E, ×100). (J) The keloidal type with broad, thick and irregularly oriented collagen bundles (H&E, ×100). |
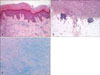 | Fig. 8Histopathologic features of dermatofibroma. (A) Typical epidermal change of dermatofibroma-induced hyperkeratosis, acanthosis and basal layer hyperpigmentation (H&E, ×100). (B) Lymphoid nodule formation in the cellular type (H&E, ×40). (C) Mucin deposition in the cellular type (alcian blue, ×40). |
References
1. Rahbari H, Mehregan AH. Adnexal displacement and regression in association with histiocytoma (dermatofibroma). J Cutan Pathol. 1985. 12:94–102.

2. Requena L, Fariña MC, Fuente C, Piqué E, Olivares M, Martín L, et al. Giant dermatofibroma. A little-known clinical variant of dermatofibroma. J Am Acad Dermatol. 1994. 30:714–718.
3. De Hertogh G, Bergmans G, Molderez C, Sciot R. Cutaneous cellular fibrous histiocytoma metastasizing to the lungs. Histopathology. 2002. 41:85–86.

4. Niemi KM. The benign fibrohistiocytic tumours of the skin. Acta Derm Venereol Suppl (Stockh). 1970. 50:Suppl. 63. 1–66.
5. Iwata J, Fletcher CD. Lipidized fibrous histiocytoma: clinicopathologic analysis of 22 cases. Am J Dermatopathol. 2000. 22:126–134.
6. Weedon D. Weedon's skin pathology. 2010. 3rd ed. Philadelphia: Churchill Livingstone;821–825.
7. Santa Cruz DJ, Kyriakos M. Aneurysmal ("angiomatoid") fibrous histiocytoma of the skin. Cancer. 1981. 47:2053–2061.

8. Zelger BW, Steiner H, Kutzner H. Clear cell dermatofibroma. Case report of an unusual fibrohistiocytic lesion. Am J Surg Pathol. 1996. 20:483–491.
10. Antal A, Zelger B, Reifenberger J, Niehues T, Feyen O, Megahed M, et al. Multiple eruptive myxoid dermatofibromas: report of first case and review of literature. Br J Dermatol. 2007. 157:382–385.

11. Kuo TT, Hu S, Chan HL. Keloidal dermatofibroma: report of 10 cases of a new variant. Am J Surg Pathol. 1998. 22:564–568.
12. Schwob VS, Santa Cruz DJ. Palisading cutaneous fibrous histiocytoma. J Cutan Pathol. 1986. 13:403–407.

13. Kuo TT, Chan HL. Ossifying dermatofibroma with osteoclast-like giant cells. Am J Dermatopathol. 1994. 16:193–195.

14. Wilson Jones E, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989. 120:185–195.

15. McKee PH, Calonje E, Granter SR. Pathology of the skin: with clinical correlations. 2005. 3rd ed. Philadelphia: Elsevier Mosby;1742–1752.
16. Ahn SK, Lee NH, Kang YC, Choi EH, Hwang SM, Lee SH. Histopathologic and immunohistochemical findings of dermatofibromas according to the clinical features and duration. Korean J Dermatol. 2000. 38:500–505.
17. Kim YJ, Seo SJ, Kim MN, Hong CK, Song KY, Ro BI. A clinical and histopathological study of dermatofibromas. Korean J Dermatol. 1996. 34:769–774.
18. Vilanova JR, Flint A. The morphological variations of fibrous histiocytomas. J Cutan Pathol. 1974. 1:155–164.

19. Sohn IB, Hwang SM, Lee SH, Choi EH, Ahn SK. Dermatofibroma with sclerotic areas resembling a sclerotic fibroma of the skin. J Cutan Pathol. 2002. 29:44–47.

20. Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990. 14:1156–1164.
21. Chang SE, Choi JH, Sung KJ, Moon KC, Koh JK. Subcutaneous dermatofibroma showing a depressed surface. Int J Dermatol. 2001. 40:77–78.

22. Schoenfeld RJ. Epidermal proliferations overlying histiocytomas. Arch Dermatol. 1964. 90:266–270.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download