Abstract
Background
So far, studies on the inter-relationship between Malassezia and Malassezia folliculitis have been rather scarce.
Objective
We sought to analyze the differences in body sites, gender and age groups, and to determine whether there is a relationship between certain types of Malassezia species and Malassezia folliculitis.
Methods
Specimens were taken from the forehead, cheek and chest of 60 patients with Malassezia folliculitis and from the normal skin of 60 age- and gender-matched healthy controls by 26S rDNA PCR-RFLP.
Results
M. restricta was dominant in the patients with Malassezia folliculitis (20.6%), while M. globosa was the most common species (26.7%) in the controls. The rate of identification was the highest in the teens for the patient group, whereas it was the highest in the thirties for the control group. M. globosa was the most predominant species on the chest with 13 cases (21.7%), and M. restricta was the most commonly identified species, with 17 (28.3%) and 12 (20%) cases on the forehead and cheek, respectively, for the patient group.
Malassezia folliculitis, as with seborrheic dermatitis, affects sites where there is an enhanced activity of sebaceous glands such as the face, upper trunk and shoulders. These patients often present with mild pruritus or follicular rash and pustules without itching1,2. It usually occurs in the setting of immuno-suppression such as the use of steroids or other immunosuppressants, chemotherapeutic agents, bone marrow transplantation and diabetes. The etiology is presumed to be overgrowth of Malassezia yeasts, which normally exist as harmless resident flora of the skin1,2.
Malassezia yeasts, which are known to be the culprits of seborrheic dermatitis, pityriasis versicolor and neonatal pustulosis, have recently taken the center stage. Their central role as the aggravating factor of atopic dermatitis has become the center of attention for the past several years and the number of research articles and case reports on this has shown a rapid increase3-6. However, despite a high level of interest, there have been only scarce studies on the inter-relationship between Malassezia and Malassezia folliculits to this point.
In response to the growing demands, the authors of this study, with the aid of 26S rDNA PCR-RFLP, sought to find clues on this inter-relationship by showing how the frequency and distribution of Malassezia yeasts vary according to age, gender and the body site, and we also wanted to compare the results with those of a healthy control group.
The patient group consisted of sixty Malassezia folliculitis patients (30 males and 30 females; 20 in their teens, 20 in their twenties and 20 in their thirties) who were diagnosed with the disorder from July of 2005 to December of 2009 at Konkuk University Hospital, the outpatient Department of Dermatology, and these 60 patients were matched with sixty healthy controls. Excluded from the study were those patients who were on systemic adrenocorticoid therapy, phototherapy or antifungal agents within the past two months, as well as those who had been treated with topical antifungal agents within the past month and/or with topical corticosteroid within one week. All the subjects were instructed to avoid the use of moisturizers and facial cleansing on the day of examination, and informed consent was obtained from each individual after providing a thorough explanation of the possible physical and psychological adverse outcomes that may arise during the course of the study. This study was conducted in strict abidance with the principles of the Declaration of Helsinki.
Leeming-Notman agar medium was prepared by mixing glycerol monoesterate (BDH, Poole, UK) 0.5 g, bacteriological peptone (Oxoid, Hampshire, UK) 20 g, glucose (Oxoid) 5 g, yeast extract (Oxoid) 0.1 g, ox bile (Merck, Darmstadt, Germany) 4 g, agar No.1 (Oxoid) 12 g, Tween 60 (Yakuri, Osaka, Japan) 0.5 ml and glycerol (Tedia, Fairfield, CA, USA) 1 ml with one liter of distilled water and then sterilizing it for twenty minutes at 121℃. Following the sterilization process, cycloheximide (Sigma, St Louis, MO, USA) 200 mg and chloramphenicol (Sigma) 50 mg were added, followed by 5 ml of non-skim milk treated at a super-high temperature (Konkuk Dairy, Seoul, Korea). After thorough mixing, the solution was spread evenly on a petri dish and kept refrigerated until use. Washing solution was prepared by dissolving NaH2PO42H2O 1.17 g into 100 ml of distilled water; 85 ml of which was taken and mixed with Na2HPO4 10.6 g and 1,000 ml of distilled water. The pH was adjusted to 7.9, and 1 ml of Triton X100 was added to the solution before sterilizing it at 121℃ for twenty minutes and then refrigerating it.
Specimens were harvested from forehead, cheek and chest, and the specimens were sampled by a scrub-wash technique, based on the method suggested by Williamson and Kligman7. A stainless tube with an interior area of 4.909 cm2 was set on the selected part of the skin (forehead, cheek and chest) and then 1 ml of detergent (0.01% NaH2PO42H2O, 1.01% Na2HPO4, 0.1% Triton X-100 [pH 7.9]) was added to the tube. After rubbing the skin with a glass rod for one minute, the sample was removed using a pipette and stored in a different container. Then, 1 ml of the detergent was added to the stainless tube, and the specimen was repetitively sampled and added to the first sample. One hundred microliters of the sampled specimen was then mixed with 900µl of the detergent, and 100µl was taken from the mixture, evenly applied on the Leeming-Notman medium and cultured at 34℃ for 14 days.
For DNA extraction and PCR analysis of the skin isolates, we adopted colony PCR analysis8, which was developed for extracting the DNA directly from a colony of a PCR tube and for amplification of 26S rDNA at the same time instead of using the direct genomic DNA extraction methods. A colony of Malassezia yeast was removed, transferred to a PCR tube and warmed 3 times a day for 1 minute each time in a double boiler using a microwave; the tube was then immersed in ice water. The PCR reaction mixture (0.25 mM deoxynucleoside triphosphate, 10X PCR buffer, 5X Q buffer, 0.5µM primers, 1.25 U Hot StarTaq polymerase, 20 mM MgSO4) was added and vortex mixing was performed. Then, PCR was performed immediately using a Mastercycler 5333 (Eppendorf, Hamburg, Germany). To amplify the 26S rDNA, a primer that is capable of amplifying all 11 standard strains at once was chosen. The sequence was as follows: forward, 5'-TAACAAGGATTCCCCTAGTA-3' and reverse, 5'-ATTACGCCAGCATCCTAAG-3'. The conditions in the early stage of the reaction were 95℃ for 14 minutes for pre-denaturation, 94℃ for 45 seconds for denaturation, 55℃ for 45 seconds for annealing, 72℃ for 1 minute for extension of 40 cycles, and 72℃ for 7 minutes for the last extension. The amplified DNA was visualized by electrophoresis on 1.5% (w/v) agarose gel with ethidium bromide (0.5µg/ml) staining and using 1X TAE migrating buffer (pH 8.0, 40 mM Tri-acetate 1 mM EDTA).
After checking the amplified 26S rDNA, the product of PCR was purified using an Accu-Prep PCR purification kit (Bioneer, Daejeon, Korea). For the 26S rDNA RFLP analysis of Malassezia yeasts, two restriction enzymes were used: Hha I (Takara Biomedicals, Otsu, Japan) and BtsF51 (SibEnzyme, Novosibirsk, Russia). The restriction enzyme reaction was conditioned with 10X PCR buffer and 10 U restriction enzyme, and the reaction mixture included 7.5µl of the PCR product. After three hours of reaction at 37℃, electrophoresis was performed in TAE buffer on 3.5% (w/v) NuSieve GTG agarose gel (FMC, Rockland, ME, USA). The gel was then stained with ethidium bromide, and the size and number of DNA fragments were measured by a UV transilluminator for analysis of the RFLP patterns (Fig. 1).
Among the 60 Malassezia folliculitis patients, Malassezia yeasts were isolated in 106 of 180 (58.9%) samples collected from the forehead, cheek and chest, and five species of Malassezia (M. restricta, M. sympodialis, M. furfur, M. globosa and M. dermatis) were identified. The same five species were also identified in the healthy controls. By age, the teens group (age 11~20) had the highest rate of detection at 63.3% (38 of 60), followed by the thirties group (37 of 60, 61.7%) and the twenties group (31 of 60, 51.7%). In the control group, the thirties group had the highest rate (45 of 60, 75%) (Table 1). According to site, the chest had the highest detection rate at 63.3% (38 of 60), followed by the forehead (37 of 60, 61.7%) and the cheek (31 of 60, 51.7%). In the control group, the rate was highest for the forehead (51 of 60, 85%) (Table 2).
By age, M. restricta was the most frequently isolated species in the teens (23.3%) and twenties groups (25.0%), while M. sympodialis was the most predominant species in the thirties group (30%). In contrast, in the control group, M. globosa was the most predominant species in the teens (28.3%) and twenties (33.3%) groups, while M. sympodialis was most commonly isolated in the thirties group (25%) (Fig. 2). Statistical significance was detected in the teens and twenties groups, but not in the thirties group (Table 3).
Overall, M. restricta was the most frequently isolated species (37 of 180, 20.6%). On the forehead, M. restricta was found in 28.3% of the cases, M. sympodialis was found in 20% and M. globosa was found in 6.7%. On the cheek, M. restricta was isolated in 20% of the cases, M. sympodialis was isolated in 16.7% and M. globosa was isolated in 10.0%. On the chest, M. restricta was isolated in 13.3%, M. globosa was isolated in 21.7% and M. sympodialis was isolated in 18.3%, while M. furfur was found at a significantly lower frequency, with a 10% detection rate. In the control group, M. globosa was most commonly isolated in total (48 of 180, 26.7%) (Fig. 3). However, among the three body sites, a statistically significant difference was detected only on the forehead (Table 4).
Malassezia yeasts are lipophilic fungi and they are considered to be normal flora of the skin, and they are isolated in 75~80% of healthy subjects1,2. Previously named as Pityrosporum in 1889 by Baillon9, Malassezia yeasts are now classified as dimorphic fungi after it was revealed that they harbor hyphae. Even though morphologic variation had been described from earlier times, only M. pachydermatis had been classified as Malassezia species, other than M. furfur. However, as the morphologic, immunologic, physiologic and molecular biological studies have progressed, the need for a new classification system of the species was put forth, and in 1996 Guého et al.10 re-classified the yeasts into seven species (M. furfur, M. obtusa, M. globosa, M. slooffiae, M. sympodialis, M. pachydermatis and M. restricta) based on the morphologic, microscopic, physiologic and molecular biological characteristics. Also, four new species called M. dermatis, M. japonica, M. nana and M. yamatoensis, have recently been introduced from Japan, and in Europe, two additional species called M. caprae and M. equina have been identified, and the yeasts are now classified into thirteen species11-15.
Malassezia yeasts affect the follicles and the keratin layer, where there is an abundant amount of essential free fatty acids and triglycerides16. Malassezia-associated dermatologic disorders can be divided into the following two groups17: the first group includes disorders of skin that are directly caused by Malassezia, and this includes Malassezia folliculitis and pityriasis versicolor. The second category can be described as aggravation of pre-existing skin diseases by growth of Malassezia, and the examples include seborrheic dermatitis, atopic dermatitis and psoriasis.
Malassezia yeasts may turn pathogenic under specific conditions. These conditions include high temperature, high humidity and internal factors such as the long-term use of corticosteroids and immunosuppressants, chemotherapeutic agents, bone marrow transplantation, AIDS, leukemia and diabetes, which elicit the overgrowth of otherwise harmless Malassezia yeasts1,2,18. According to the previous studies, M. globosa is commonly isolated in pityriasis versicolor19-22, and although the reports on seborrheic dermatitis have varied22-24, M. restricta seems to be the most commonly found species in Korea25. The studies on Malassezia folliculitis by Jang et al.26 revealed that M. restricta and M. globosa are the commonly isolated species. Our study revealed that depending on the site, M. restricta or M. globosa is the most common strain that resides in a lesion.
Since Weary27 first reported on the correlation between Pityrosporum and folliculitis and acne, Potter et al.28 presented a detailed report on Malassezia and Malassezia folliculitis. Malassezia folliculitis is a mycotic skin disorder in which an itchy follicular rash and pustules develop on the upper trunk and shoulders where there is abundant secretion by sebaceous glands. It is prevalent during summertime in tropical climates, and a closed environment is a crucial factor for its pathogenesis. Various factors such as the use of tetracyclines or related antibiotics, systemic or local administration of corticosteroids, diabetes, bone marrow transplantation and Cushing syndrome are thought to cause overgrowth of Malassezia yeasts among the resident flora, and this manifests with the aforementioned symptoms1,2. Because rash and pustules may develop on the face and alongside the trunk and shoulders, folliculitis must be differentiated from deep-seated acne. It can be distinguished from acne by the fact that it develops mostly on the trunk and that there are no comedones29.
The diagnosis of Malassezia folliculitis is made by its characteristic clinical manifestations, direct smear microscopy, histopathological examination and the fact that it responds to antifungal therapy. The majority of cases can be treated by local application of antifungal agents, yet some cases may require the oral administration of ketoconazole, itraconazole or fluconazole6.
On comparing the healthy controls and the Malassezia folliculitis patients, five species of Malassezia, namely M. restricta, M. sympodialis, M. furfur, M. globosa and M. dermatis were identified in each group. Also, the most commonly isolated species in the Malassezia folliculitis patients group was M. restricta, while M. globosa was the most common in the healthy control group. The detection rate was the highest in the teens and thirties subgroups in the Malassezia folliculitis group, and in the control group, the thirties age subgroup showed the highest rate.
Among the lesions sampled on the chest, M. globosa was isolated in 13 cases (21.7%), followed by M. sympodialis in 11 cases (18.3%), and on the forehead and cheek, M. restricta was the most predominant species with 17 cases (28.3%) and 12 cases (20.0%), respectively, followed by M. sympodialis with 12 cases (20%) and 10 cases (16.7%), respectively. The outcome differed somewhat from that of the report by Jang et al.26, which stated that M. restricta was found in 4 cases (20%), M. globosa was found in 2 cases (10%) and M. furfur was found in 1 case (5%) on the face, while M. globosa was only predominant with a total of 12 cases (65%) on the neck, chest, flank and sacral area. These disparities may be attributable to the difference in the methods of the two studies, i.e., morphological analysis, such as the size, surface contour, color and shape of the Malassezia colonies, and molecular analysis, such as PCR and RFLP. In addition, other factor such as the differences in the number of subjects, the length of the study, the temperature and the humidity at the time of measurement and various technical factors may also have played roles.
We were able to obtain statistically significant differences between the patient and control groups on the forehead and for the teens and twenties subgroups.
For the results, we identified that the detection rate of M. globosa was lower and that of M. sympodialis was higher, except in the twenties group, as compared to that of the control group.
Although M. restricta was most commonly detected in the Malassezia folliculitis patients, the lower detection rate of M. globosa and the higher detection rate of M. sympodialis suggest that M. sympodialis might possess a pathogenic potential; however, the selection of the subjects and the research methods, along with environmental factors, have to be taken into consideration. According to the report by Akaza et al.30, in which the identification rate of the Malassezia species on the back, upper chest, and neck was compared between 32 Malassezia folliculitis patients (average age: 31±11 years) and 40 controls (average age: 33±7 years), M. sympodialis was detected at a not insignificant frequency in all the specimens. The microflora of the skin, such as M. sympodialis, are known to stimulate keratinocytes to secrete certain specific cytokines and to induce inflammation of hair follicles, thus leading to folliculitis31. Further, Kim et al.32 have reported that M. sympodialis was identified as the main causative agent on the face of a neonate with Malassezia folliculitis. M. sympodialis was found on mycological examination of the folliculitis lesions of renal cell carcinoma patients who underwent treatment with erlotinib, an anticancer agent33, and this further increases the possibility of this strain being pathogenic. However, considering that there have been reports, such as those by Crespo and Delgado6 and Rhie et al.34, that the main causative agents of Malassezia folliculitis are normal flora of the skin other than M. sympodialis, and that other strains such as M. furfur, had been isolated from the neonatal Malassezia folliculitis lesions on the face3, and given the outcome of this study in which the rate of identifying M. sympodialis in the twenties control group was lower than that in the patient group, the possibility of this strain being pathogenic may be questioned.
Therefore, in order to identify the Malassezia species that play a major role in the pathogenesis and aggravation of Malassezia folliculitis, a large-scale quantitative analysis in future studies conducted on a larger patient pool and more variable sites of lesions may be needed.
Figures and Tables
Fig. 1
PCR-RFLP patterns of the 26S rDNA PCR, as digested with Hha I (A) and BstF51 (B), of the 11 Malassezia standard strains. Lanes: M: molecular marker, 1: M. furfur (KCTC 7743), 2: M. sympodialis (KCTC 7985), 3: M. globosa (CBS 7966), 4: M. restricta (KCTC 7848), 5: M. slooffiae (KCTC 17431), 6: M. pachydermatis (KCTC 17008), 7: M. japonica (CBS 9432), 8: M. nana (JCM 12085), 9: M. dermatis (JCM 11348), 10: M. obtusa (KCTC 7847), 11: M. yamatoensis (CBS 9725).
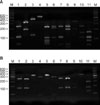
Fig. 2
The identified Malassezia species from the Malassezia folliculitis group and as compared with those from the control group by age.

Fig. 3
The identified Malassezia species from the Malassezia folliculitis group as compared with those from the healthy control group by the body site.

References
1. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1828–1830.
2. Ahn KJ. Taxonomy of the genus Malassezia. Korean J Med Mycol. 1998. 3:81–88.
3. Rapelanoro R, Mortureux P, Couprie B, Maleville J, Taïeb A. Neonatal Malassezia furfur pustulosis. Arch Dermatol. 1996. 132:190–193.
4. Kim KS, Kye YC, Kim SN, Ahn KJ. A case of neonatal Malassezia pustulosis induced by Malassezia sympodialis. Korean J Dermatol. 2000. 38:1427–1429.
5. Ljubojević S, Skerlev M, Lipozencić J, Basta-Juzbasić A. The role of Malassezia furfur in dermatology. Clin Dermatol. 2002. 20:179–182.
6. Crespo Erchiga V, Delgado Florencio V. Malassezia species in skin diseases. Curr Opin Infect Dis. 2002. 15:133–142.
7. Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965. 45:498–503.

8. Kim SM, Lim SH, Jung BR, Lee YW, Choe YB, Ahn KJ. The application of colony PCR in the molecular biological analysis of Malassezia yeasts. Korean J Med Mycol. 2007. 12:180–188.
9. Baillon HE. Traité de botanique médicale cryptogamique, suivi du tableau du droguier de la Faculté de médecine de Paris. 1889. Paris: Doin.
10. Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek. 1996. 69:337–355.

11. Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002. 40:1363–1367.

12. Hirai A, Kano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, et al. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol. 2004. 54:623–627.

13. Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003. 41:4695–4699.

14. Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, et al. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Immunol. 2004. 48:579–583.

15. Cabañes FJ, Theelen B, Castellá G, Boekhout T. Two new lipid-dependent Malassezia species from domestic animals. FEMS Yeast Res. 2007. 7:1064–1076.
16. Porro MN, Passi S, Caprill F, Nazzaro P, Morpurgo G. Growth requirements and lipid metabolism of Pityrosporum orbiculare. J Invest Dermatol. 1976. 66:178–182.
17. Terui T, Kudo K, Tagami H. Cutaneous immune and inflammatory reactions to Malassezia furfur. Nippon Ishinkin Gakkai Zasshi. 1999. 40:63–67.
18. Yohn JJ, Lucas J, Camisa C. Malassezia folliculitis in immunocompromised patients. Cutis. 1985. 35:536–538.
19. Ahn KJ. Malassezia species cultured from the lesions of pityriasis versicolor. Korean J Dermatol. 1997. 35:736–743.
20. Ahn KJ, Kim KJ, Yi GJ. Efficacy of one-week regimen of itraconazole for pityriasis versicolor. Korean J Med Mycol. 1999. 4:124–130.
21. Crespo Erchiga V, Ojeda Martos A, Vera Casaño A, Crespo Erchiga A, Sanchez Fajardo F. Malassezia globosa as the causative agent of pityriasis versicolor. Br J Dermatol. 2000. 143:799–803.
22. Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000. 38:337–341.

23. Pechère M, Krischer J, Remondat C, Bertrand C, Trellu L, Saurat JH. Malassezia spp carriage in patients with seborrheic dermatitis. J Dermatol. 1999. 26:558–561.
24. Parry ME, Sharpe GR. Seborrhoeic dermatitis is not caused by an altered immune response to Malassezia yeast. Br J Dermatol. 1998. 139:254–263.

25. Lee YW, Kang HJ, Ahn KJ. Malassezia species cultured from the lesions of seborrheic dermatitis. Korean J Med Mycol. 2001. 6:70–76.
26. Jang SJ, Choi YB, Ahn KJ. Malassezia species cultured from the lesions of Malassezia folliculitis. Korean J Med Mycol. 2003. 8:55–62.
27. Weary PE. Pityrosporum ovale: observations on some aspects of host-parasite interrelationship. Arch Dermatol. 1968. 98:408–422.
28. Potter BS, Burgoon CF Jr, Johnson WC. Pityrosporum folliculitis. Report of seven cases and review of the Pityrosporum organism relative to cutaneous disease. Arch Dermatol. 1973. 107:388–391.

29. Bäck O, Faergemann J, Hörnqvist R. Pityrosporum folliculitis: a common disease of the young and middle-aged. J Am Acad Dermatol. 1985. 12:56–61.
30. Akaza N, Akamatsu H, Sasaki Y, Kishi M, Mizutani H, Sano A, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009. 47:618–624.

31. Watanabe S, Kano R, Sato H, Nakamura Y, Hasegawa A. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Invest Dermatol. 2001. 116:769–773.

32. Kim HJ, Lee MH, Ahn KJ. A case of neonatal Malassezia pustulosis identified as Malassezia sympodialis. Korean J Med Mycol. 2001. 6:229–231.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download