Abstract
Objective
The aim of this study was to identify β-catenin-regulated genes in cultured human hair outer root sheath (ORS) cells.
Methods
Primary cultured ORS cells were transduced with recombinant adenovirus expressing N-terminal truncated β-catenin (constitutive active form), and β-catenin-regulated genes were identified.
Results
Overexpression of the constitutively active form of β-catenin led to induction of Sox9 expression at both mRNA and protein levels. To investigate the potential role of Sox9, we made the recombinant adenovirus expressing green fluorescent protein-tagged Sox9, and then transduced into cultured ORS cells. Interestingly, Sox9 induced the expression of keratin 15, increased the proliferation of ORS cells in vitro, and enhanced colony-forming activity.
During embryogenesis, hair follicles are formed from the ectoderm and the underlying mesoderm, following a reciprocal exchange of signals between epithelial and mesenchymal cells1. One important inductive signal for hair follicles is the Wnt/β-catenin system. Wnts are a family of secreted glycoproteins that serve as extracellular signaling factors. In canonical Wnt/β-catenin signaling cascade, binding of Wnt ligands to their cognate membrane receptors leads to the inactivation of β-catenin degradation complex, resulting in the stabilization of cytoplasmic β-catenin. Once accumulated, β-catenin translocates to the nucleus and interacts with Lef-1/TCF family of DNA-binding proteins to generate a functional transcription factor complex2.
The fundamental role of β-catenin in hair follicle formation has been confirmed by several outstanding studies. For example, Gat et al.3 showed that mice expressing a stabilized β-catenin controlled by an epidermal promoter undergo a process resembling de novo hair follicle morphogenesis. In addition, a conditional deletion of β-catenin in the skin after hair follicle formation leads to the complete loss of hair after first cycle4. These results clearly demonstrate that β-catenin is necessary for hair follicle formation.
Although the importance of Wnt/β-catenin signaling in the morphogenesis and growth cycle of hair follicles is well recognized, the downstream effectors of β-catenin have not yet been clearly elucidated. In this study, we identified Sox9 as a β-catenin-regulated gene in cultured human outer root sheath (ORS) cells, and demonstrated that Sox9 has potential importance in the regulation of hair follicle homeostasis.
Hair follicles were isolated from scalp specimens according to a previously reported method5. Hair follicles were incubated with 0.25% trypsin, 0.02% ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered saline (PBS) for 10 min. Hair follicles were then vigorously pipetted to obtain single cell populations. The dissociated cells were rinsed in Dulbecco's modified Eagle's medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), and centrifuged for 5 min at 200 g. ORS cells were then resuspended in keratinocyte-serum free medium (K-SFM) supplemented with epidermal growth factor (EGF) and bovine pituitary extract (Gibco) and seeded onto culture dish. Cultures were maintained at 37℃ in a humidified atmosphere of 5% CO2.
To identify β-catenin-regulated genes, we transduced ORS cells with adenovirus expressing N-terminal 87-amino acid truncated β-catenin (ΔN87βCat) and performed cDNA microarray using 27 K human cDNA microarray slides (GenomicTree, Daejeon, Korea)5. Microarray slides were analyzed using GenePix Pro 4.0 software (Axon Instruments, Union City, CA, USA) and GeneSpring 7.2 software (Agilent Technologies, Redwood City, CA, USA).
Scalp specimens were obtained from plastic surgery, in accordance with the ethical committee approval process of Chungnam National University Hospital. Specimens were embedded in paraffin. Sections of specimens were dewaxed, rehydrated, and washed three times with PBS. After treatment with proteinase K (1 mg/ml) for 5 min at 37℃, sections were treated with H2O2 for 10 min at room temperature, blocked in 0.1% Tween-20, 1% bovine serum albumin in PBS for 20 min, and followed by reaction with anti-Sox9 antibody (Abcam, Cambridge, MA, USA) for 1 h. Sections were incubated sequentially with peroxidase-conjugated secondary antibodies (Upstate, Lake Placid, NY, USA) and visualized with Chemmate envision detection kit (Dako, Carpinteria, CA, USA).
Total RNA was isolated from cultured ORS cells using Easy-blue RNA extraction kit (Intron, Daejeon, Korea). Two µg of total RNA was reverse transcribed with moloney-murine leukaemia virus reverse transcriptase (ELPIS biotech, Daejeon, Korea). Aliquot of RT mixture was subjected to polymerase chain reaction (PCR) cycles with primers for Sox9 (5'-GCAACCGGATCCATGAATCTCCTGGACCCCTT and 5'-GCAACCGGATCCTCAAGGTCGAGTGAGCTGT). The amplified full-length cDNA for Sox9 was subcloned into pENT/GFP vector that has attL sites for site-specific recombination with a Gateway destination vector (Invitrogen, Carlsbad, CA, USA). The replication-incompetent adenoviruses were created using Virapower adenovirus expression system (Invitrogen), according to a previously reported method6. Briefly, site-specific recombination between entry vector and adenoviral destination vector was achieved by LR clonase (Invitrogen). The resulting adenoviral expression vector was then transfected into 293A cells using Lipofectamine 2000 (Invitrogen). Cells were grown until 80% cytopathic effect was seen, then harvested for preparation of recombinant adenovirus.
To evaluate gene expression, 2µg of total RNA was reverse transcribed and then subjected to PCR cycles with specific primer sets. The primers used in the study are as follows, cyclophilin 5'-CTCCTTTGAGCTGTTTGCAG and 5'-CACCACATGCTTGCCATCCA, β-catenin 5'-TGCAGTTCGCCTTCACTATG and 5'-CTGCACAAACAATGGAATGG, SOX9 5'-AGACAGCCCCCTATCGACTT and 5'-TAGGAGGGGCTGTAGTGTGG, keratin-15 5'-GCCTGGTTCTTCAGCAAGAC and 5'-GGGA CGTTTCTCCTGCAATA.
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 1 mM EDTA, 0.1% NP-40 and 0.2 mM PMSF). After vigorous pipetting, extracts were centrifuged for 15 min at 13,000 rpm. Total protein was measured using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples were run on 10% SDS-polyacrylamide gels, transferred onto nitrocellulose membranes and incubated with appropriate antibodies overnight at 4℃ with gentle agitation. Blots were then incubated with peroxidase-conjugated secondary antibodies for 1 h at room temperature, and visualized by enhanced chemiluminescence (Intron). Anti-β-catenin, anti-GFP, anti-keratin15 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), and anti-actin antibodies (Sigma, St. Louis, MO, USA) were used.
For determination of cell growth, [3H]thymidine uptake assay was performed. ORS cells were seeded in 60-mm culture dish and transduced with adenovirus overnight. Cells were replenished with fresh medium containing 1µCi of [3H]thymidine (Amersham, Buckinghamshire, UK). Following incubation, cells were washed twice with PBS and incubated with 0.1 N NaOH at room temperature. Radioactivity in cell lysates was measured by liquid scintillation counter.
ORS cells were transduced with adenovirus overnight, replenished with fresh medium, and cultured for 2 days. Cells were trypsinized and counted using a hemocytometer. Approximately 1,000 cells were then resuspended in K-SFM supplemented with EGF and bovine pituitary extract, and then seeded onto 6-well plates. Cells were incubated for 2 weeks and stained with Crystal violet (Sigma).
β-catenin consists of a 130 amino-terminal domain, 12 imperfect repeats of 42 amino acids (arm repeats), and a carboxy-terminal domain of 100 amino acids (Fig. 1A). It has been demonstrated that N-terminal truncation of β-catenin makes it resistant to proteosomal degradation, rendering it constitutively stabilized and thereby functioning as a transcription factor7. To express the constitutively stabilized β-catenin in ORS cells, we made the recombinant adenovirus expressing ΔN87βCat. After adenoviral transduction into ORS cells, the expression of the exogenously introduced gene was determined by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis (Fig. 1B and C).
We attempted to identify the genes regulated by β-catenin using cDNA microarray5, and found that Sox9 was induced by overexpression of constitutive active form of β-catenin, at both mRNA and protein levels (Fig. 1B and C). To investigate the putative role of Sox9, we first examined the expression of Sox9 in human hair follicle. As shown in Fig. 2, Sox9 expression was detected in the outer layer of middle portion of ORS. In a previous report, Sox9 expression was restricted in ORS of bulge region in mice, suggesting an important role for the formation of hair stem cell compartment8. Although it has been well established that stem cells reside in the bulge region of rodent hair follicle, the location of stem cells in human hair follicles is believed to be broader than in mouse hair follicles9. Therefore, we speculated that Sox9 also had a functional role linked to stemness in human hair follicle cells.
To further investigate the role of Sox9, we made the recombinant adenovirus expressing green fluorescent protein (GFP)-tagged Sox9. After adenoviral transduction into ORS cells cultured in vitro, the expression of Sox9 was detected in the nucleus of ORS cells (Fig. 3A). We then determined the effect of Sox9 on the expression of stem cell marker keratin 15 by RT-PCR and Western blot. Interestingly, overexpression of Sox9 led to the induction of keratin 15 at both mRNA and protein levels (Fig. 3B and C). However, overexpression of Sox9 did not influence β-catenin expression (Fig. 3C). Based on these result, we hypothesized that Sox9 has a role in maintenance of stemness in human hair follicle. One easy method for checking stemness in vitro is to determine the cell growth rate, because stem cells are prone to grow more rapidly than transient amplifying cells and/or differentiated cells under culture conditions10. As shown in Fig. 4A, overexpression of Sox9 led to the increase of PCNA, a proliferation marker. Consistent with this result, thymidine uptake assays clearly showed that Sox9 increased the proliferation of ORS cells. In vitro clonogenicity is another well-established assay for self-renewal potential11. Consistent with the above findings, ORS cell colonies were highly increased by Sox9 expression (Fig. 5).
The ORS cells are ectoderm-derived keratinocytes that are known to have specified roles in maintaining hair follicle structure and regulating hair growth cycle. In particular, ORS keratinocytes located in the bulge area in mice are well known to have several properties consistent with being stem cells12. In human hair follicles, stem cells are also located in ORS cells; however, their distribution is believed to be wider than that of mouse hair follicles9. The hair stem cells contribute largely to the cyclic property of hair growth. During the telogen to anagen transition, a cluster of stem cells in bulge becomes activated to proliferate, and then move downward. In this process, these cells are also committed to differentiate into follicular keratinocytes to form actively hair-producing follicle. Although the precise mechanism underlying the activation of bulge stem cells remains to be elucidated, it has been demonstrated that transient activation of β-catenin signaling is sufficient to trigger the active growth phase of hair cycle in mice13.
In the present study, we overexpressed stabilized β-catenin in ORS cells using adenovirus, and found that Sox9 was induced by activation of β-catenin signaling. Sox9 (SRY (sex determining region Y)-box 9) belongs to the high mobility group box transcription factor family, and involved in the control of the male-determining pathway14. In other systems, Sox9 has been shown to be regulated by β-catenin. For instance, Wnt signaling pathway promotes chodrocyte differentiation in a Sox9-dependent manner15. Another example shows that Sox9 protein is expressed in the intestinal epithelium in a pattern characteristic of Wnt targets16. In our study, overexpression of the stabilized β-catenin led to an increase in Sox9 expression in ORS cells cultured in vitro. Furthermore, Sox9 induced the expression of keratin 15 and enhanced ORS cell proliferation and clonogenicity. In mouse hair follicles, Sox9 is expressed in bulge ORS and the conditional knockout of Sox9 in skin led to a defect in formation of stem cell niche8. Based on these findings, it is plausible that Sox9 is an authentic β-catenin-downstream modulator regulating human hair follicle homeostasis.
Despite the fact that Sox9 is a downstream effector molecule of β-catenin in ORS cells, it is unlikely that Sox9 is sufficient for anagen induction. In our preliminary experiments, intradermal injection of adenovirus expressing Sox9 at the catagen stage (7-week-old C57BL/6 mice) failed to induce the catagen to anagen transition (data not shown). The precise regulation mechanism by which Sox9 modulates hair cell homeostasis needs to be investigated further.
In summary, our results suggest that Sox9 is a functional downstream effector of β-catenin in ORS cells and has potential importance in the regulation of hair follicle homeostasis.
Figures and Tables
Fig. 1
(A) Structure of β-catenin. The N-terminus has several phosphorylation sites, which are important for regulating the stability of β-catenin. Central domain consists of 12 arm repeats, which allow for overlapping interaction with multiple binding partners. The C-terminus functions as a transcriptional activation domain. N-terminal 87-amino acid truncated β-catenin (ΔN87β Cat) is depicted under the wild-type β-catenin. (B) Outer root sheath cells were transduced with adenovirus expressing ΔN87βCat at 10 multiplicity of infection. The mRNA level was verified by reverse transcription-polymerase chain reaction. (C) After adenoviral transduction, protein level was verified by Western blot. Upper band represents the endogenous β-catenin, while lower band represents the N-truncated β-catenin (arrow). Adenovirus expressing lacZ was used as negative control.
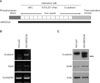
Fig. 2
Immunohistochemical staining of Sox9 (×100). Paraffin section of scalp specimen was incubated with anti-Sox9 antibody, and then sequentially incubated with HRP-conjugated secondary antibody. Intense expression of Sox9 is detected in the outer layer of outer root sheath in middle portion of hair follicle.
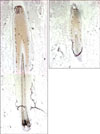
Fig. 3
(A) Expression of exogenous Sox9 in outer root sheath (ORS) cells. ORS cells were transduced with adenovirus expressing GFP-tagged Sox9 (GFP-Sox9) at 10 multiplicity of infection (MOI). Adenovirus expressing GFP was used as negative control. Twenty-four hours after adenoviral transduction, cells were observed under the fluorescent microscopy. (B) Effect of Sox9 on the expression of stem cell marker in ORS cells. Cells were transduced with adenovirus expressing GFP-Sox9 at the indicated MOI. The mRNA level was verified by reverse transcription-polymerase chain reaction. (C) Cellular extracts were prepared, and the protein level for stem cell marker was verified by Western blot. Actin was used as a loading control.
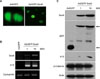
Fig. 4
Effect of Sox9 on cell proliferation. (A) Cells were transduced with adenovirus expressing GFP-Sox9 at the indicated multiplicity of infection. The proliferation marker PCNA was examined by Western blot. (B) Thymidine uptake assay. After adenoviral transduction, cells were received [3H]thymidine-containing medium. After a 3 day incubation, cells were lysed and radioactivity was measured using liquid scintillation counter. Data are expressed as % control and SEM from three independent experiments. *p<0.01.
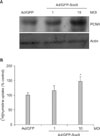
Fig. 5
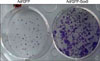
Effect of Sox9 on clonogenicity. ORS cells were transduced with adenovirus (10 multiplicity of infection). After 2 day incubation, cells were detached from the culture dish and 1,000 cells were re-seeded onto new culture dish. After 2 week incubation, colony formation was verified by staining with Crystal violet.
References
3. Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998. 95:605–614.

4. Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001. 105:533–545.

5. Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, a β-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009. 54:6–11.

6. Kim JH, Choi DK, Lee SS, Choi SJ, Kim CD, Yoon TJ, et al. Enhancement of keratinocyte differentiation by rose absolute oil. Ann Dermatol. 2010. 22:255–261.

7. Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated β-catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998. 141:765–777.

8. Vidal VP, Chaboissier MC, Lützkendorf S, Cotsarelis G, Mill P, Hui CC, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005. 15:1340–1351.

9. Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006. 116:249–260.

10. Yang JS, Lavker RM, Sun TT. Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. J Invest Dermatol. 1993. 101:652–659.

11. Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005. 102:14677–14682.

12. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990. 61:1329–1337.

13. Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003. 17:1219–1224.

14. Barrionuevo F, Scherer G. SOX E genes: SOX9 and SOX8 in mammalian testis development. Int J Biochem Cell Biol. 2010. 42:433–436.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download