Abstract
Background
Atrichia with papular lesions (APL) is a rare inherited disease characterized by early onset of total hair loss, followed by papular lesions over the extensor areas of the body. Recently, mutations in the human hairless (HR) gene have been implicated in its pathogenesis. The identification of mutations in the HR gene is important for differentiating between APL and alopecia universalis (AU).
Objective
We compared the HR genes of patients with presumed AU who showed minimal or no response to treatment with the HR genes of healthy controls.
Methods
The subjects were 11 patients with presumed AU who had not responded to treatments. Fifty healthy people were included as controls for molecular analysis. To screen for mutations, polymerase chain reaction was performed.
Results
DNA analysis identified a novel heterozygous G-to-A transition at nucleotide position 191 in exon 5. The mutation was not found in the controls, other AU patients, or any unaffected family members except for the patients' mother and maternal grandfather, who were heterozygous HR gene carriers.
Atrichia with papular lesions (APL) is a rare form of irreversible complete loss of hair on the scalp and entire body, and is known to be inherited in an autosomal recessive manner1-4. Clinically, APL is characterized by total hair loss, including scalp, face, axilla, and body hair, during the first few months of life. This is followed by the appearance of multiple follicular papules that consist histopathologically of keratin-filled cysts and are especially prevalent on the face and extensor surfaces of the extremities.
Recently, molecular analysis has been used to diagnose a patient with APL, and studies of numerous APL families have implicated mutations of the hairless (HR) gene in the pathogenesis of this disorder4-6. Several mutations that map to chromosome 8p12 have been identified, and increasing numbers of HR gene mutations are being reported.
Most APL patients have homozygous HR gene mutations, and have been discovered in consanguineous families living within a restricted geographical region, implying that these mutations became fixed owing to a high degree of consanguinity1-3. However, the number of sporadic cases occurring in offspring of unrelated parents has been increasing7-11. Sporadic patients with non-consanguineous parents have been misdiagnosed with alopecia universalis (AU) frequently, as APL and AU are clinically very similar. After unsuccessful treatment for AU, they were subsequently diagnosed as APL by molecular analysis5,12. Thus, the identification and evaluation of HR gene mutations in patients with total hair loss are important for differentiating between APL and AU.
In this study, the HR genes of AU patients who showed little or no response to treatments for restoring hair growth were examined and compared with the HR genes of normal healthy controls, and a novel missense mutation of the HR gene was identified in a Korean boy who finally diagnosed with APL.
The study examined 11 patients presumed to have AU who were seen in the dermatology clinic at Busan Paik Hospital, Busan, Korea and had little or no response to systemic and topical hair restoration treatments. The molecular analyses of HR genes in the AU patients and 50 normal healthy controls were compared. Among the 11 patients with presumed AU, one male was diagnosed with APL based on his HR gene analysis. Subsequently, his immediate family and several close relatives were also included in this study. He had no family history of APL and no history of parental consanguinity.
The proband was a 4-year-old boy who was born with nearly normal scalp hair but sparse eyebrows and eyelashes. The scalp hair began to shed soon after birth and never regrew. He progressed to complete and permanent hairlessness by the age of 3 years. He did not respond to standard systemic and topical immunotherapy for AU, which included potent steroids and diphencyprone (DPCP). Multiple follicular papules began to appear on his face, chest, back, elbows, and knees when he was 3 years old, and the eruptions gradually increased in number. His nails, teeth, and sweating were normal, and there was no evidence of ectodermal dysplasia.
We obtained blood samples from 11 AU patients (including proband), family members of the proband, and 50 normal healthy controls. To confirm APL diagnosis, punch biopsies from the right occipital region of the scalp and the left thigh, and a follicular papule from the left knee of the proband were obtained under local anesthesia. The biopsy samples were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 4-µm-thick sections, and stained with hematoxylin and eosin using standard protocols.
To detect mutations in the human HR gene, all exons and splice junctions of the HR gene from each subject's genomic DNA were amplified by the polymerase chain reaction (PCR), purified using GeneAll® Expin™ Gel SV (GeneAll Biotechnology, Seoul, Korea), and sequenced directly in an ABI Prism 3100 automated sequencer, using a ABI Prism Dye Terminator Cycle Sequencing Ready Reaction kit (SolGent, Daejeon, Korea). The PCR conditions have been described elsewhere5. The mutation was identified by comparisons of the HR sequences among the AU patients and control subjects, using the ClustalX multiple sequence alignment program (Strasburg, France). The mutation was verified in the patient by comparing the BseRI (New England Biolabs, Beverly, MA, USA) restriction pattern of his HR gene with those of several family members (his parents, maternal and paternal grandparents, an aunt, two uncles, and a brother), the AU patients, and the control individuals.
At the time of examination, the proband had only two mature black hairs remaining, in the right occipital region. We also observed absence of body hair, and sparse eyebrows and eyelashes (Fig. 1A, B). More than 100 small papules were present over the scalp, face, chest, back, both elbows, and both knees (Fig. 1A, C, D). There were also several fine, whitish, hypopigmented streaks on the scalp. On careful examination of the patient, a novel clinical sign that has not been described in previous reports of APL was observed, namely, hypopigmented macules on the entire body, especially on both thighs (Fig. 1E, F). The patient had no abnormalities of the nails, teeth, sweating, or laboratory findings, including vitamin D metabolites. He also has no major developmental or growth deficiencies.
Only two mature black hairs were present in the right occipital region of the proband. The skin biopsy specimen from the scalp showed a single intact mature hair and a markedly reduced number of hair follicles. A few vellus hairs and some granulomatous changes were observed around an anagen hair follicle. There was mild perivascular lymphocytic infiltration limited to the infundibulum and isthmus level with no inflammation around the hair bulb (Fig. 2A).
The skin biopsy specimen of multiple tiny whitish macules on the thigh showed the loss of hair follicles with remnant arrector pili muscles. There was no remarkable change in pigmentation, including melanin and melanocytes, in the basal layer of the epidermis (Fig. 2B).
A biopsy of a follicular papule on the knee showed a keratinous cyst containing fine laminated eosinophilic keratinous material. The cyst wall consisted of several layers of keratinocytes showing gradual flattening, and a granular layer was present in the cystic epithelium. The histopathological findings of the papule on the knee were compatible with an epidermal inclusion cyst (Fig. 2C).
The proband showed a novel G-to-A transition at nucleotide position 191 in exon 5, resulting in the replacement of a glycine residue by a glutamate; this was called a G64E missense mutation (Fig. 3). The G64E mutation eliminated a restriction site for the endonuclease BseRI and was used as a screening assay in 9 relatives, 10 other AU patients, and 50 Korean control individuals. The proband's mother and maternal grandfather were heterozygous carriers of the G64E mutation (Fig. 4). But, the chromosomes containing the HR gene in all of the other AU patients and healthy controls were wild type. Therefore, this mutation was not a polymorphism. The absence of this mutation in the control chromosomes and the nature and severity of the clinical findings indicate that this was a pathogenic mutation in this patient.
Relatively little is known about the mechanism of APL. Although hair follicle development appears to be normal in APL, a defect in the follicular cycling system must induce hair loss because normal hair is present at birth but is subsequently lost during the first few months of life. Panteleyev et al.13 postulated that hair regrowth in APL is disrupted because the lower follicle disintegrates during catagen and the normal juxtaposition of epithelial stem cells in the bulge and mesenchymal cells in the dermal papilla of the hair follicle is not re-established during telogen14. This interferes with mesenchymal-epithelial interactions, which are considered to be necessary for re-entry into anagen, and atrichia is the result14. Consequently, the architecture of hair follicles with short catagen stages, including those of vibrissae, eyelashes, and eyebrows, may not be disrupted sufficiently during catagen, and these hairs may be preserved in APL patients15. Recent studies have suggested that HR protein acts as a nuclear receptor co-repressor in concert with the vitamin D receptor gene and represses the expression of WISE protein, which inhibits Wnt signaling16,17. The follicular cysts seen in APL patients may arise from follicular remnants that survive after the follicle breaks apart during catagen13,18. As the cyst epithelium expresses keratin K17, which normally occurs in the outer root sheath of hair follicles, cysts in APL patients appear to arise from the bulge of the hair follicle13,19. The regression of these follicular papules in older patients has been reported, although the mechanism is unclear4.
Approximately 34 mutations of the HR gene, including the one discovered in this study, have been reported6,10-12,20-22. These include missense, nonsense, deletion, insertion, splice-site, and compound heterozygous mutations10,11,23-25. This study reports a novel G-to-A transition at nucleotide 191 in exon 5, resulting in the replacement of a glycine residue by a glutamate residue. This new missense mutation in the HR gene is called the G64E mutation. This particular mutation has not been reported previously, and this is only the second case report of APL occurring in a Korean family.
Although different mutations of the HR gene have been reported, the clinical manifestations of APL exhibit little variation, and most APL patients have the same degree of clinical severity. There do not appear to be any particular "hot spots" or common locations for mutation in the HR gene or any genotype-phenotype connections in APL patients. Also, there is no information on HR protein levels in affected patients, particularly the actual amount of altered HR protein expressed in the skin. Therefore, to diagnose APL, it is important to sequence all coding regions, including exon-intron junctions, of the entire HR gene.
APL is usually inherited in an autosomal recessive manner. Most reported cases of APL have been discovered in consanguineous families living within a restricted geographical region. However, there are some reports of APL patients with compound heterozygous mutations in small non-consanguineous families7-11. In present study, the novel missense mutation was not inherited as an autosomal recessive, as the patient's mother and maternal grandfather were heterozygous carriers of the G64E mutation, whereas his father had the wild-type HR gene. There are three possible explanations for proband's homozygosity: 1) Proband's father is not his biological father. 2) The father has a large heterozygous deletion in the same region which cannot be recognized by sequencing and which was inherited by the proband. 3) The proband had a spontaneous mutation of the same type as his mother's (G-to-A transition at position 191 in exon 5), which seems to be less likely. It seems that isolated cases of APL may be more common than previously thought, because of a lack of awareness and the misdiagnosis of APL as AU or vitamin-D-dependent rickets, which may present as atrichia in the first few months of life and as follicular cysts in the first few years of life11,17.
On careful examination of this patient, hypopigmented macules and streaks were observed. Hypopigmented streaks on the scalp in APL patients have been reported before6. However, follicular hypopigmented macules over the entire body, especially on both thighs, have not been reported, and may be novel clinical signs of APL. The mechanism and pathogenesis of the hypopigmented streaks or macules in APL is unknown, but it may be that the HR gene mutation could play a role in the development of the follicular hypopigmented macules.
In summary, we report a new case of APL in a nonconsanguineous family that was diagnosed based on clinical findings and molecular data and involved a novel G-to-A transition at nucleotide 191 in exon 5. In addition, a novel clinical sign of APL was observed, namely, follicular hypopigmented macules on the entire body, especially on both thighs. This particular mutation has not been reported previously, and this is only the second case report of APL occurring in a Korean family. These data extend our knowledge of mutations in the HR gene, and suggest that children with presumed AU in non-consanguineous families that do not respond to standard treatment modalities may warrant testing for HR gene mutations to avoid unnecessary treatments.
Figures and Tables
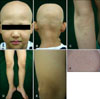 | Fig. 1Clinical appearance of the APL patient. (A, B) There is complete loss of hair on the scalp, and the eyebrows and eyelashes are sparse. (A, C, D) There are follicular papules on the face, elbows, and knees. (E) Note the multiple hypopigmented follicular macules on the thighs. (F) These are also visible on a dermoscopic view. |
 | Fig. 2Histopathological findings of the only mature hair in the occipital region, the hypopigmented macules on the right thigh, and a follicular papule on the left knee. (A) On the scalp, there are a few vellus hairs and mild perivascular lymphocytic infiltration around the infundibulum and isthmus of the only mature hair. There is no inflammation around the hair bulb (H&E, ×40). (B) There is a loss of hair follicles on the thigh, with remnant arrector pili muscles. There are no marked changes in pigmentation, including melanin and melanocytes, of the basal layer of the epidermis (H&E, ×40). (C) The keratinous cysts in the skin over the knee contain laminated fine eosinophilic keratinous material and a granular layer in the cystic epithelium, compatible with an epidermal inclusion cyst (H&E, ×40). |
ACKNOWLEDGMENTS
We thank the patient, his family and relatives, and all of the control subjects for participating in this study.
References
1. Fredrich HC. Zur kenntnis der kongenitale hypotrichosis. Dermatol Wochenschr. 1950. 121:408–410.
2. Damste TJ, Prakken JR. Atrichia with papular lesions; a variant of congenital ectodermal dysplasia. Dermatologica. 1954. 108:114–121.

4. Zlotogorski A, Martinez-Mir A, Green J, Lam H, Panteleyev AA, Sinclair R, et al. Evidence for pseudodominant inheritance of atrichia with papular lesions. J Invest Dermatol. 2002. 118:881–886.

5. Cichon S, Anker M, Vogt IR, Rohleder H, Pützstück M, Hillmer A, et al. Cloning, genomic organization, alternative transcripts and mutational analysis of the gene responsible for autosomal recessive universal congenital alopecia. Hum Mol Genet. 1998. 7:1671–1679.

6. Zlotogorski A, Panteleyev AA, Aita VM, Christiano AM. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2002. 118:887–890.

7. Indelman M, Bergman R, Lestringant GG, Peer G, Sprecher E. Compound heterozygosity for mutations in the hairless gene causes atrichia with papular lesions. Br J Dermatol. 2003. 148:553–557.

8. Paller AS, Varigos G, Metzker A, Bauer RC, Opie J, Martinez-Mir A, et al. Compound heterozygous mutations in the hairless gene in atrichia with papular lesions. J Invest Dermatol. 2003. 121:430–432.

9. Henn W, Zlotogorski A, Lam H, Martinez-Mir A, Zaun H, Christiano AM. Atrichia with papular lesions resulting from compound heterozygous mutations in the hairless gene: A lesson for differential diagnosis of alopecia universalis. J Am Acad Dermatol. 2002. 47:519–523.

10. Ashoor GG, Greenstein RM, Lam H, Martinez-Mir A, Zlotogorski A, Christiano AM. Novel compound heterozygous nonsense mutations in the hairless gene causing atrichia with papular lesions. J Dermatol Sci. 2005. 40:29–33.

11. Yip L, Horev L, Sinclair R, Zlotogorski A. Atrichia with papular lesions: a report of three novel human hairless gene mutations and a revision of diagnostic criteria. Acta Derm Venereol. 2008. 88:346–349.
12. Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998. 279:720–724.

13. Panteleyev AA, van der Veen C, Rosenbach T, Muller-Rover S, Sokolov VE, Paus R. Towards defining the pathogenesis of the hairless phenotype. J Invest Dermatol. 1998. 110:902–907.

14. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990. 61:1329–1337.

15. Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001. 104:233–245.

16. Thompson CC, Sisk JM, Beaudoin GM 3rd. Hairless and Wnt signaling: allies in epithelial stem cell differentiation. Cell Cycle. 2006. 5:1913–1917.

17. Wang J, Malloy PJ, Feldman D. Interactions of the vitamin D receptor with the corepressor hairless: analysis of hairless mutants in atrichia with papular lesions. J Biol Chem. 2007. 282:25231–25239.
18. Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM. Molecular and functional aspects of the hairless (HR) gene in laboratory rodents and humans. Exp Dermatol. 1998. 7:249–267.

19. McGowan KM, Coulombe PA. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J Invest Dermatol. 2000. 114:1101–1107.

20. Kruse R, Cichon S, Anker M, Hillmer AM, Barros-Nunez P, Cantu JM, et al. Novel hairless mutations in two kindreds with autosomal recessive papular atrichia. J Invest Dermatol. 1999. 113:954–959.
21. Zlotogorski A, Hochberg Z, Mirmirani P, Metzker A, Ben-Amitai D, Martinez-Mir A, et al. Clinical and pathologic correlations in genetically distinct forms of atrichia. Arch Dermatol. 2003. 139:1591–1596.

22. Ahmad W, Zlotogorski A, Panteleyev AA, Lam H, Ahmad M, Faiyaz ul Haque M, et al. Genomic organization of the human hairless gene (HR) and identification of a mutation underlying congenital atrichia in an Arab Palestinian family. Genomics. 1999. 56:141–148.

23. Betz RC, Indelman M, Pforr J, Schreiner F, Bauer R, Bergman R, et al. Identification of mutations in the human hairless gene in two new families with congenital atrichia. Arch Dermatol Res. 2007. 299:157–161.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download