Abstract
Acute generalized exanthematous pustulosis is clinically characterized by fever, pruritus and an acute pustular eruption. It can be described as having an abrupt onset and then spontaneous resolution occurs shortly after the start of symptoms, and there is usually only a single episode. Most cases have been triggered by the ingestion of drugs. Diltiazem hydrochloride is a calcium channel blocker that is commonly used for treating hypertension and angina. This drug was found to be the responsible agent in our current patient. There have been 9 such case reports in the English medical literature, yet this is the first such report in the Korean medical literature. We present the case of a 51-year-old male who experienced an acute generalized exanthematous pustulosis due to diltiazem hydrochloride and we review the relevant literature.
Acute generalized exanthematous pustulosis (AGEP) is an uncommon eruption that is characterized by acute, extensive formation of sterile pustules on an erythematous background and this is combined with fever and peripheral blood leucocytosis1. Most cases have been triggered by the ingestion of antibiotics and particularly the beta-lactams and macrolides2. There is usually rapid resolution of the eruption after drug withdrawal. Herein, we report on a patient who developed AGEP shortly after commencing treatment with the calcium channel blocker diltiazem hydrochloride. To the best of our knowledge, this is the first case of diltiazem-induced AGEP to be reported in the Korean medical literature.
A 51-year-old male presented to the dermatology clinic with a 2-day history of non-pruritic multiple pustular lesions that mainly affected the trunk. The patient had a history of chest discomfort 6 months previously. His symptoms continued to develop and he was then examined with echocardiography, a tilt test and coronary angiography. After being examined, the patient was diagnosed has having variant angina and he was started on oral diltiazem hydrochloride (Herben SR® once and Dilteran SR® once daily). His other concurrent medication was oral aspirin (100 mg once daily) for the previous 5 months. The eruption appeared four days after starting the oral diltiazem. He instantly stopped taking the oral diltiazem and aspirin. He had no previous drug allergy or family history of skin disease.
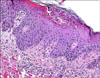
Physical examination of the patient revealed an erythematous, maculopapular eruption with small, non-follicular pustules that mainly affected the trunk (Fig. 1). Over the next 2 days, the eruption became more confluent with widespread pustulation on the scalp, face and extremities and he also had a fever (38℃). The rest of his physical examination was unremarkable. The routine hematological and biochemical investigations, including liver function tests, were within the normal limits except for a leukocytosis with a neutrophil predominance and a raised erythrocyte sedimentation rate (ESR) of 33 mm/hr. The virological and bacteriological cultures of the pustules remained sterile. Histopathological examination from an excised pustule showed subcorneal pustules of the epidermis with mild spongiosis. In the dermis, there was papillary edema in addition to a dense perivascular infiltrate of lymphocytes and neutrophils (Fig. 2). The patient was treated with oral prednisolone (20 mg twice daily) and oral antihistamine, which resulted in gradual improvement of his rash and pustules within 7 days along with a markedly decreased white blood cell count. In addition, he received topical steroid lotion on the affecting area. The skin lesions settled down and the oral prednisolone was tapered to 10mg once daily. After 7 days his skin lesions were almost diminished except for localized desquamation and reticulate post-inflammatory hyperpigmentation. There has been no recurrence of the eruption during 2 months of follow-up.
In 1991 Roujeau et al.1 described AGEP as an acute pustular dermatosis characterized by fever (a temperature above 38℃) and a widespread exanthematous eruption associated with hundreds of small nonfollicular superficial pustules. The blood neutrophil count is usually elevated, and mild renal impairment occurs in approximately 30% of the cases; both of these abnormalities disappear with improvement of the eruption1. Other lesions that may be seen in patients with this dermatosis are erythema multiforme-like target lesions, vesicles, blisters or purpura3. Mucous membrane involvement, if it occurs, is generally mild and limited to one location. Systemic involvement, including lymphadenopathy, hepatosplenomegaly and pharyngitis, may occur. The bacteriological and fungal examinations of the pustules are negative. In most patients the rash often develops within 24~48 h of drug exposure on the face or in the flexures, and then it rapidly disseminates and lasts about 1~2 weeks1,3. In addition, the EuroSCAR study observed 2 different patterns for the onset time after exposure to the offending agent. The reaction to antibiotics usually occurred within 1 day, whereas for all other drugs the median time to the onset of a reaction was 11 days4. The cause for this difference and whether it represents prior sensitization remain undetermined. By removing the offending agent and with supportive therapy, healing occurs within 2~3 weeks in most cases.
AGEP has been described most often in association with a drug etiology. In an analysis of 63 cases, a responsible drug was found in 87% of the patients with this dermatosis and beta-lactam antibiotics were implicated as the causative agents in 80% of these patients1. The long list of responsible agents includes macrolide antibiotics, cephalosporins, doxycycline, isoniazid, furosemide, itraconazole, captopril, allopurinol and hydroxychloroquine. There are several theories regarding the pathogenesis of AGEP. Britschgi et al.5 suggested a T-cell-mediated mechanism. In this model, the infiltrating T cells release extended amounts of CXCL8, which is a neutrophil attracting chemokine, resulting in neutrophilic inflammation. In addition to the CXCL8, T cells also induce interferon γ, interleukin 4 and interleukin 5, and these all provoke eosinophilic and neutrophilic aggregation. Helper T cells are thought to form vesicles, cytotoxic T cells are involved in local tissue destruction and neutrophils create the sterile pustules5-7. In this view, drug-specific T cells expressing CXCL8 play a critical role in the development of AGEP, and there may be an initial sensitization period4-7. Moreau et al.8 proposed that AGEP is a delayed type of hypersensitivity reaction. Another possible mechanism is the production of antigen-antibody complexes induced by an infection or drug that activates the complement system, which in turn leads to neutrophil chemotaxis2.
The histopathologic findings shows spongiform subcorneal intraepidermal or subepidermal pustules filled with neutrophil leukocytes or eosinophils, epidermal spongiosis, subepidermal edema, polymorphous perivascular infiltrates and focal necrosis of keratinocytes. Leukocytoclastic vasculitis was reported in some cases1.
Diltiazem hydrochloride is a calcium channel blocker that was introduced in the UK in 1984 and it is now commonly used for treating hypertension and angina. The incidence of reported cutaneous side-effects has ranged from 65 per million prescriptions to about 1% of patients taking this drug9. A wide range of dermatological manifestations has been documented, including urticaria, erythema multiforme, toxic epidermal necrolysis, photosensitive erythroderma, allergic vasculitis and hyperplastic gingivitis. There have been only nine reports in the English literature to date of AGEP following diltiazem therapy10-15.
The mean number of days of therapy with diltiazem to the onset of lesions was 8.4 days. The skin lesions fade at a mean of 10 days after stopping diltiazem10-15. In our patient, the skin eruption began 4 days after the initiation of diltiazem and it cleared within 14 days of discontinuing the drug and receiving oral prednisolone. The previous reported cases resolved completely following drug withdrawal. In some cases, the patients received additional treatment with systematic corticosteroids.
Patch testing has been suggested to be a useful investigation for patients with disseminated cutaneous reactions to diltiazem, and Sousa-Basto et al.16 reported positive patch test results in all three patients with suspected drug hypersensitivity. Unfortunately, we did not perform a patch test. However, diltiazem was probably the agent responsible for our patient's skin rash based on a review of the medical history. There has been no recurrence of the eruption for 2 months.
Here we report on the first case of diltiazem-induced AGEP in the Korean medical literature.
Figures and Tables
References
1. Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991. 127:1333–1338.

2. Beylot C, Doutre MS, Beylot-Barry M. Acute generalized exanthematous pustulosis. Semin Cutan Med Surg. 1996. 15:244–249.

3. Breathnach SM. Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Drug reaction. Rook/Wilkinson/Ebling textbook of dermatology. 1998. 6th ed. Oxford: Blackwell Science;3349–3517.
4. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP): results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007. 157:989–996.

5. Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001. 107:1433–1441.

6. Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002. 2:325–331.

7. Beltraminelli HS, Lerch M, Arnold A, Bircher AJ, Haeusermann P. Acute generalized exanthematous pustulosis induced by the antifungal terbinafine: case report and review of the literature. Br J Dermatol. 2005. 152:780–783.

8. Moreau A, Dompmartin A, Castel B, Remond B, Leroy D. Drug-induced acute generalized exanthematous pustulosis with positive patch tests. Int J Dermatol. 1995. 34:263–266.

9. Stern R, Khalsa JH. Cutaneous adverse reactions associated with calcium channel blockers. Arch Intern Med. 1989. 149:829–832.

10. Lambert DG, Dalac S, Beer F, Chavannet P, Portier H. Acute generalized exanthematous pustular dermatitis induced by diltiazem. Br J Dermatol. 1988. 118:308–309.

11. Nishimura T, Yoshioka K, Katoh J, Mizuno N, Kono T, Tanii T, et al. Pustular drug eruption induced by diltiazem. Skin Res. 1991. 33:Suppl. 10. 251–254.
12. Wittal RA, Fischer GO, Georgouras KE, Baird PJ. Skin reactions to diltiazem. Australas J Dermatol. 1992. 33:11–18.

13. Janier M, Gerault MH, Carlotti A, Vignon MD, Daniel F. Acute generalized exanthematous pustulosis due to diltiazem. Br J Dermatol. 1993. 129:354–355.

14. Wakelin SH, James MP. Diltiazem-induced acute generalised exanthematous pustulosis. Clin Exp Dermatol. 1995. 20:341–344.

15. Jan V, Machet L, Gironet N, Martin L, Machet MC, Lorette G, et al. Acute generalized exanthematous pustulosis induced by diltiazem: value of patch testing. Dermatology. 1998. 197:274–275.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download