Abstract
Matridex® is an injectable skin filler that's composed of a mixture of cross linked hyaluronic acid and dextranomer particles, and it was recently developed for soft tissue augmentation. To the best of our knowledge, there have been few previous reports on complications associated with Matridex. We report here on a delayed inflammatory reaction to an injection of Matridex in the glabellar fold, and this developed five weeks after the injection and it lasted more than a year. The patient was treated with oral doxycycline and intralesional injection of triamcinolone acetonide; this resulted in almost complete resolution of the lesion. The patient should be informed of the potential complications with the use of injectable fillers before treatment, for it could lead to undesirable aesthetic consequences.
An increasing number of injectable filler substances have been developed in the recent decades for soft tissue augmentation. Matridex® (BioPolymer, Siershahn, Germany) is a biodegradable, injectable filler that's composed of cross-linked hyaluronic acid and cross-linked dextran microspheres. These form microparticles with a positively charged surface and a diameter of approximately 80~120 µm. Hyaluronic acid is a naturally occurring glycosaminoglycan polysaccharide that's composed of alternating residues of the monosaccharides D-glucuronic acid and N-acetyl-D-glucosamine; it is found in the mammalian extracellular matrix and has no species specificity. Hyaluronic acid is used as a vehicle to support the relatively large dextran molecules in a spherical hydrodynamic unit owing to its viscoelasticity. Hyaluronic acid has an immediate volume-enhancing effect through its considerable water-binding ability. The molecular network structure of hyaluronic acid also helps to evenly distribute the dextran molecules after injection into tissues. Dextran microspheres are known to stimulate the formation of new collagen fibers. Eppley et al.1 have reported that dextran beads attract macrophages to their positively charged surfaces, and that macrophages release TGF-beta and interleukins, which in turn stimulate fibroblasts.
We report here on a delayed inflammatory reaction due to the injection of Matridex in the glabellar fold, and this reaction developed five weeks after the injection and it lasted for more than 1 year. To the best of our knowledge, there has been only one previous report of complication related to Matridex2.
A 56-year-old Korean female presented to the Dermatology Department with a painful firm erythematous nodule in the glabellar fold. The patient reported that she had received intradermal injections of Matridex in the glabellar folds for correction of facial wrinkles 14 months previously at a private dermatology clinic. No pretreatment skin testing for evidence of hypersensitivity to the filler had been performed. Several days after the injections, redness and intermittent swelling were noted on the right-sided glabellar fold, but this improved within 1 week. Five weeks after the treatment, the patient developed a tender erythematous firm nodule on the right-sided glabellar fold that tended to wax and wane in size and firmness. Treatment with intralesional hyaluronidase injection was attempted, but the patient reported little improvement.
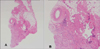
When we first examined the patient, she presented with a solitary indurated erythematous nodule with a smooth surface in the right-sided glabellar fold (Fig. 1). Other than the skin lesion, there were no remarkable findings on the physical examination. She had no specific past medical history or family history. A biopsy was performed under a presumptive diagnosis of foreign body reaction.
The histopathological examination showed a moderate lymphohistiocytic infiltration involving the muscle layer and the sub-muscle layer. These changes were accompanied by fibrosis that was most prominent in the submuscle layer (Fig. 2).
The patient was treated with oral doxycycline 100 mg twice a day for 8 weeks and then once a day for 4 weeks. She also received a total of three intralesional injections of triamcinolone acetonide (5 mg/ml) with a 4-week interval, which resulted in flattening and softening of the lesion (Fig. 3).
Matridex was first introduced in Europe in 2004. It contains a biodegradable carrier substance, hyaluronic acid, which has an immediate volume-enhancing effect, and cross-linked dextran microspheres, which stimulate collagenesis and give structure to the facial correction, resulting in a more permanent and long-lasting effect. However, hyaluronic acid degrades within 1 year and cross-linked dextran degrades within 1~2 years. Thus, the volume augmentation effects of Matridex are likely to be of short duration. The manufacturers of Matridex suggest that the products have no or minimal allergy risk and that allergy testing is therefore unnecessary. There is only one previous report of complication associated with Matridex, and this was seen in a 43-year-old woman on both cheeks and periorbital areas 4 weeks after the injection of Matridex at these sites2. In that previous case, the histopathology revealed a diffuse suppurative granulomatous reaction surrounding 2 different types of exogenous materials: one was arranged in filamentous structures and the second was composed of spherical particles2. Lemperle et al.3 have previously reported that Reviderm intra® (Rofil Medical International, Breda, Netherlands), which is a suspension of dextran microspheres in hyaluronic acid and it is similar to Matridex, induced a marked foreign body reaction after dermal injection into the volar forearm. They suggested that the immediate swelling and redness that persisted for 10 days were possibly due to the toxic effects of free dextranomers. At 1 month after the injection, a palpable deep dermal nodule had developed and it lasted for 6 months. Histological examination of the lesion revealed large numbers of macrophages and giant cells surrounding the dextran beads and the further study revealed that the hyaluronic acid carrier had separated from the beads.
Hyaluronic acid has no organ or species specificity and theoretically it poses no risk of allergy. However, a large study of the hyaluronic acid fillers Restylane and Hyalaform found that 0.42% of the patients experienced delayed inflammatory skin reactions4. The cause of late inflammation after intradermal injection of hyaluronic acid is not yet known, but it has been suggested that the cause could be allergic in nature5. Given that the hyaluronic acid in Matridex is derived from fermentation involving bacteria, it could be due to proteic impurities. It could also be due to the chemical modification of the hyaluronic acid structure during the stabilization process. Micheels6 have reported the presence of circulating antibodies against hyaluronic acid in patients after several injections, which also supports the allergy hypothesis. Transient inflammatory reactions after intradermal injections of hyaluronic acid, such as severe redness, bruising, swelling, pain and tenderness, have been reported in 3~5% of patients7,8. The local effects of puncture trauma, as well as the hygroscopic properties of the filler being used, may cause these reactions. These effects usually resolve within 2~3 days and they only rarely persist for several weeks7,8.
Histopathological examination of the patient in the present case revealed a delayed, non-acute inflammatory reaction that predominantly involved lymphohistiocytic infiltration and fibrosis. There were no identifiable exogenous materials or granuloma formations. However, according to the classification of foreign body reactions established by Duranti et al.7, the histopathologic feature in our patient could be classified as a grade 1 foreign body reaction (slight inflammatory reaction with a few inflammatory cells). Considering the previously reported cases and our case, a reaction to the dextran microspheres or the hyaluronic acid in Matridex is quite possible.
Repetitive injectable cortisone or oral cortisone with tapering the dose over time and topical tacrolimus, together with time for the symptoms to resolve, have been the treatment options for persistent nodules that have developed after injections of soft tissue filler. More recently, Brody9 has reported the use of hyaluronidase to treat a hyaluronic acid-related granulomatous foreign body reaction. However, our patient had previously not responded to treatment with intralesional injections of hyaluronidase. We decided to administer doxycycline, based on previous reports of the clinical anti-inflammatory and immunomodulatory responses to doxycycline in the case of granulomatous reaction induced by soft tissue filler10 and also in other granulomatous diseases such as sarcoidosis11.
Our case report showed a delayed inflammatory reaction to the locally injected Matridex, which was composed of cross-linked dextran molecules with hyaluronic acid. The patient was treated with oral doxycycline and intralesional injection of triamcinolone acetonide, and the result was almost complete resolution of the lesion. Although rare, Matridex injection for cosmetic purposes may produce a delayed inflammatory reaction, and this can lead to undesirable aesthetic consequences. With the increasing availability of diverse soft tissue fillers for cosmetic purposes, physicians should be aware of this delayed complication from injectable fillers that are composed of cross-linked dextran microspheres and hyaluronic acid.
Figures and Tables
Fig. 1
(A) A solitary indurated erythematous nodule with a smooth surface in the right-side glabellar fold. (B) The lateral view of the lesion.

References
1. Eppley BL, Summerlin DJ, Prevel CD, Sadove AM. Effects of a positively charged biomaterial for dermal and subcutaneous augmentation. Aesthetic Plast Surg. 1994. 18:413–416.

2. Massone C, Horn M, Kerl H, Ambros-Rudolph CM, Giovanna Brunasso AM, Cerroni L. Foreign body granuloma due to Matridex injection for cosmetic purposes. Am J Dermatopathol. 2009. 31:197–199.

3. Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003. 27:354–367.

4. Lowe NJ, Maxwell CA, Lowe P, Duick MG, Shah K. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001. 45:930–933.

5. Friedman PM, Mafong EA, Kauvar AN, Geronemus RG. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002. 28:491–494.

6. Micheels P. Human anti-hyaluronic acid antibodies: is it possible? Dermatol Surg. 2001. 27:185–191.

7. Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatol Surg. 1998. 24:1317–1325.
8. Manna F, Dentini M, Desideri P, De Pita O, Mortilla E, Maras B. Comparative chemical evaluation of two commercially available derivatives of hyaluronic acid (hylaform from rooster combs and restylane from streptococcus) used for soft tissue augmentation. J Eur Acad Dermatol Venereol. 1999. 13:183–192.

9. Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005. 31:893–897.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download