Abstract
Oral hairy leukoplakia (OHL) is caused by the reactivation of a previous Epstein-Barr virus (EBV) infection in the epithelium of the tongue. Most lesions are characterized by corrugated whitish patches on the lateral border of the tongue. It is frequently associated with AIDS, but cases in patients with other immunosuppressed states have also been reported. In leukemia patients, OHL is rarely encountered, and appears only after chemotherapy. We report a case of OHL which occurred as a presenting sign of acute myeloid leukemia (AML) in a previously healthy 15-year-old child. A 15-year-old boy presented with a whitish patch on the left lateral border of the tongue. The biopsy specimen revealed papillomatosis, hyperkeratosis, acanthosis and ballooning degeneration in the stratum spinosum. The patient was EBV seropositive, and PCR analysis of EBV DNA in the lesional tissue was positive. After the diagnosis of OHL in dermatologic department, the patient was referred to pediatrics due to the abnormal peripheral blood smear, and was diagnosed with AML.
Oral hairy leukoplakia (OHL) was first described by Greenspan et al.1 in 1984. It is characterized by whitish patches with a corrugated or hairy surface most commonly on the lateral borders of the tongue. Its distinctive feature is produced by the proliferation of mucosal epithelium, which is caused by the reactivation of a previous Epstein-Barr virus (EBV) infection2. As the histopathology features are not pathognomonic, the demonstration of EBV in the lesional tissue is essential for a definite diagnosis3. OHL is found most commonly in patients infected with HIV and is regarded as an early sign and prognostic factor of a HIV infection4. It can also be associated with other immunosuppressed conditions, such as an organ or bone marrow transplantation, chemotherapy, hematological malignancies and the long term use of systemic steroid5,6.
Occasionally, cutaneous manifestations, such as leukemia cutis, pyoderma gangrenosum, erythema nodosum and chronic urticaria may be the first symptom or signs of leukemia. However, there are no reports of OHL being a presenting sign of leukemia7-12.
We report a case of OHL that occurred as a presenting sign of acute myeloid leukemia (AML) in a previously healthy 15-year-old child.
A 15-year-old boy presented with a whitish patch on the left lateral border of his tongue that had lasted for several months. The patient was a player in his middle school soccer team. He appeared healthy and denied any prior medical problems. He had no subjective symptoms but the lesion had recently increased in size. A physical examination revealed corrugated whitish patches on the left border of the tongue, which were so adhesive that they could not be scraped by a tongue depressor (Fig. 1). The differential diagnosis of the lesion included oral candidiasis, oral lichen planus and oral hairy leukoplakia. The laboratory tests including routine blood tests, peripheral blood smear tests, HIV and EBV antibody, were carried out. There was no fungal element on the KOH mount. The mucosal biopsy specimen revealed papillomatosis, hyperkeratosis, parakeratosis, acanthosis and ballooning degeneration in the upper stratum spinosum (Fig. 2). The high-power view revealed vacuolated cells with small, round, deeply basophilic nuclei surrounded by a narrow clear halo, which were compatible with oral hairy leukoplakia (Fig. 3). The patient was HIV seronegative on EIA but EBV seropositive (EBV anti-VCA IgG). There was a very low platelet count with 25×103/µl (normal range, 140×103/µl to 400×103/µl). While white blood cell count was adequate in number, blasts (10%) were found. The lesional tissue was examined to demonstrate an EBV infection using immunohistochemistry and in situ hybridization, but these showed doubtful results. A polymerase chain reaction (PCR) was performed to detect EBV DNA using a primer to the EBV W fragment. Positive control was the Hank-1 cell line, which is a case of B-cell lymphoma. 50 ng/dl of DNA of the lesional tissue showed a distinct band that was comparable to the positive control (Fig. 4). The lesion was diagnosed definitely as oral hairy leukoplakia.
Two days after the mucosal biopsy, the patient complained of a 2 cm-sized hematoma on the biopsy site, which was probably due to severe thrombocytopenia. The patient was referred to the pediatric department, where he was diagnosed with acute myeloid leukemia (AML M2) after a bone marrow biopsy. At that time, a poor outcome was expected because chromosomal analysis showed 8-trisomy. Induction chemotherapy was started immediately, but it failed and was followed by neutropenic fever. After a 3 month struggle against the leukemia, he died of septic shock.
The EBV is transmitted mainly through the saliva13. It is not only lymphocytotrophic but also epitheliotrophic, and can cause pharyngeal mucosal lesions including OHL and nasopharyngeal carcinoma3.
OHL usually affects both lateral borders of the tongue4. Unilateral involvement, as in our case, has been reported albeit rarely14-16. The histology findings of OHL include filiform hyperkeratosis, parakeratosis and acanthosis with a vacuolar alteration of epithelial cells4. Among them, the vacuolar alteration of prickle cells is the most characteristic feature4. The condition is either diffuse or focal, and each vacuolated cell has small, round, deeply basophilic nuclei surrounded by a narrow clear halo and by pale-staining cytoplasm4. OHL should be differentiated from white sponge nevus, frictional keratosis, lichen planus and idiopathic leukoplakia17. Among them, the clinical and histopathology findings of idiopathic leukoplakia are similar to those of OHL17. To confirm OHL, demonstration of the EBV in the lesional tissue is essential because the histopathology features on conventional optical microscopy are not pathognomonic. The EBV can be detected by immunohistochemistry, in situ hybridization, or a PCR. In situ hybridization is the most accurate, and is considered the gold standard technique in diagnosis3. In our case, the EBV was detected by PCR, and 'in situ hybridization' showed an inconclusive result. The EBV BamHI W repeat was used as the primer and the Hank-1 cell line (a B-cell lymphoma line) was used as the positive control18. As EBV DNA strains are present in the saliva of EBV-seropositive persons, there is a possible risk of contaminating the scraped tissue with saliva (false positive in PCR analysis). However, in case of using a biopsy specimen, as in the present case, PCR analysis is as specific and sensitive for the detection of EBV as in situ hybridization3. Successive treatment modalities using a range of topical agents, such as tretinoin solution, podophylline resin, gentian violet or antiviral agent, have been reported4,16,19-21.
Since the first report in 1984, OHL has been observed mainly in AIDS patients4,22-24. However, OHL has been reported in other immunosuppressed patients, including those with ulcerative colitis, pemphigus vulgaris, bullous pemphigoid, Behcet's syndrome, multiple myeloma, and leukemia5,6,16,17,25. Although rare cases of OHL in healthy people have been reported, OHL is believed to occur in immunosuppression as a rule14,15.
OHL is not regarded as a prognostic factor of diseases other than AIDS. However, previous cases of OHL with hematologic malignancies have been reported in patients after chemotherapy, whose state of immunosuppression would be more severe than a malignancy per se5,16,25. Therefore, it was assumed that the OHL might also be associated with an advanced state or poor prognosis in leukemia. In this case, trisomy 8 was observed by chromosomal analysis and the course of the disease was very rapid and poor. In AML, cytogenetics at diagnosis can have important prognostic significance, and a number of chromosomal abnormalities have been identified26. Among these abnormalities, trisomy 8 is the most common numerical aberration in AML, and its presence indicates a poor progonosis26. The early recognition of OHL is very important considering the high possibility of an underlying immunodeficiency and the potential indicator of a poor prognosis.
Various skin diseases can be associated with leukemia, and some can occur as the presenting sign of leukemia. These include leukemia cutis, pyoderma gangrenosum, erythema nodosum, and chronic urticaria7-12. However, OHL has never been reported as the presenting sign of leukemia. To our knowledge, this is the first report of OHL as a presenting sign of acute myeloid leukemia.
Figures and Tables
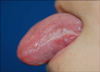 | Fig. 1Tightly adhered whitish patches are located unilaterally in the left border of the tongue showing slightly hairy projections. |
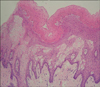 | Fig. 2Hyperkeratosis, parakeratosis, acanthosis, ballooning degeneration in the stratum spinosum and vacuolated cells with small, round, deeply basophilic nuclei surrounded by a narrow clear halo are shown (H&E, ×100). |
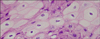 | Fig. 3Cytoplasmic halo surrounding the nucleus and peripheral margination of chromatin in the nucleus are shown (H&E, ×1,000). |
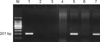 | Fig. 4Positive result shows the EBV band 201 bp region of the EBV BamHI W repeat. M: marker, Lane 1: EBV positive control, Lane 2: patient negative control, Lane 3: negative control (D.W), Lane 4: patient sample 1 (100 ng/dl), Lane 5: patient sample 1 (50 ng/dl), Lane 6: patient sample 2 (100 ng/dl), Lane 7: patient sample 2 (50 ng/dl). |
References
1. Greenspan D, Greenspan JS, Conant M, Petersen V, Silverman S Jr, de Souza Y. Oral "hairy" leucoplakia in male homosexuals: evidence of association with both papillomavirus and a herpes-group virus. Lancet. 1984. 2:831–834.
2. Woo SB. McKee PH, Calonje E, Granter S, editors. Diseases of the oral mucosa. Pathology of the skin: with clinical correlations. 2005. 3rd ed. Philadelphia: Elsevier Mosby;430–431.

3. Mabruk MJ, Flint SR, Toner M, Balluz I, Coleman D, Sullivan D, et al. In situ hybridization and the polymerase chain reaction (PCR) in the analysis of biopsies and exfoliative cytology specimens for definitive diagnosis of oral hairy leukoplakia (OHL). J Oral Pathol Med. 1994. 23:302–308.

4. Alessi E, Berti E, Cusini M, Zerboni R, Cavicchini S, Tomasini D, et al. Oral hairy leukoplakia. J Am Acad Dermatol. 1990. 22:79–86.

5. Syrjanen S, Laine P, Niemela M, Happonen RP. Oral hairy leukoplakia is not a specific sign of HIV-infection but related to immunosuppression in general. J Oral Pathol Med. 1989. 18:28–31.

6. Schiodt M, Norgaard T, Greenspan JS. Oral hairy leukoplakia in an HIV-negative woman with Behcet's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995. 79:53–56.
7. Markowski TR, Martin DB, Kao GF, Lutz L, Deng A, Gaspari AA. Leukemia cutis: a presenting sign in acute promyelocytic leukemia. Arch Dermatol. 2007. 143:1220–1221.

8. Fox LP, Geyer AS, Husain S, Grossman ME. Bullous pyoderma gangrenosum as the presenting sign of fatal acute myelogenous leukemia. Leuk Lymphoma. 2006. 47:147–150.

9. Sullivan R, Clowers-Webb H, Davis MD. Erythema nodosum: a presenting sign of acute myelogenous leukemia. Cutis. 2005. 76:114–116.
10. Callen JP, Bernardi DM, Clark RA, Weber DA. Adult-onset recalcitrant eczema: a marker of noncutaneous lymphoma or leukemia. J Am Acad Dermatol. 2000. 43:207–210.

11. Clore LS Jr, Stafford CT. Chronic urticaria as a presenting sign of hairy cell leukemia. Allergy Asthma Proc. 1999. 20:51–55.

12. Sumner WT, Grichnik JM, Shea CR, Moore JO, Miller WS, Burton CS. Follicular mucinosis as a presenting sign of acute myeloblastic leukemia. J Am Acad Dermatol. 1998. 38:803–805.

13. Young LS, Clark D, Sixbey JW, Rickinson AB. Epstein-Barr virus receptors on human pharyngeal epithelia. Lancet. 1986. 1:240–242.

14. Lozada-Nur F, Robinson J, Regezi JA. Oral hairy leukoplakia in nonimmunosuppressed patients. Report of four cases. Oral Surg Oral Med Oral Pathol. 1994. 78:599–602.
15. Lee KH, Polonowita AD. Oral hairy leukoplakia arising in an oral lichen planus lesion in an otherwise immune-competent patient. N Z Dent J. 2007. 103:58–59.
16. Nicolatou O, Nikolatos G, Fisfis M, Belegrati M, Papadaki T, Oikonomaki E, et al. Oral hairy leukoplakia in a patient with acute lymphocytic leukemia. Oral Dis. 1999. 5:76–79.

17. Greenspan D, Greenspan JS, de Souza Y, Levy JA, Ungar AM. Oral hairy leukoplakia in an HIV-negative renal transplant recipient. J Oral Pathol Med. 1989. 18:32–34.

18. Pan L, Milligan L, Michaeli J, Cesarman E, Knowles DM. Polymerase chain reaction detection of Kaposi's sarcoma-associated herpesvirus-optimized protocols and their application to myeloma. J Mol Diagn. 2001. 3:32–38.

19. Moura MD, Guimaraes TR, Fonseca LM, de Almeida Pordeus I, Mesquita RA. A random clinical trial study to assess the efficiency of topical applications of podophyllin resin (25%) versus podophyllin resin (25%) together with acyclovir cream (5%) in the treatment of oral hairy leukoplakia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007. 103:64–71.

20. Bhandarkar SS, MacKelfresh J, Fried L, Arbiser JL. Targeted therapy of oral hairy leukoplakia with gentian violet. J Am Acad Dermatol. 2008. 58:711–712.

21. Pastore L, De Benedittis M, Petruzzi M, Fiore JR, Serpico R. Efficacy of famciclovir in the treatment of oral hairy leukoplakia. Br J Dermatol. 2006. 154:378–379.

22. Greenspan D, Greenspan JS, Hearst NG, Pan LZ, Conant MA, Abrams DI, et al. Relation of oral hairy leukoplakia to infection with the human immunodeficiency virus and the risk of developing AIDS. J Infect Dis. 1987. 155:475–481.

24. Gennaro S, Naidoo S, Berthold P. Oral health & HIV/AIDS. MCN Am J Matern Child Nurs. 2008. 33:50–57.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download