Abstract
Primary cutaneous mucinous carcinoma is a rare malignant tumor that originates from the deepest portion of the eccrine sweat duct. Common sites of involvement are the face and scalp. Biopsy shows dermal epithelial cell islands embedded in mucin pools separated by fibrous septae. It is difficult to differentiate this tumor histologically from metastatic adenocarcinoma. Recurrence after excision is common but metastases are rare. We report a primary cutaneous mucinous carcinoma with neuroendocrine differentiation on the right cheek of a 63-year-old man.
Primary cutaneous mucinous carcinoma was first documented by Lennox et al.1 in 1952, and was first designated by Mendoza and Helwig2 in 1971. It is a rare malignant tumor related to the eccrine sweat duct and is commonly located on the face, especially around the eyelids, and scalp3,4. It is usually solitary and grows asymptomatically over several months or even years3. Histopathology shows a circumscribed tumor with large amounts of mucin separated by fibrous septae and containing scattered floating islands of epithelial cells.
Only eight other cases5-12 have been reported in the Korean dermatological literature (Table 1), and none of these showed neuroendocrine differentiation. We report herein a primary mucinous carcinoma with neuroendocrine differentiation on the right cheek of a 63-year-old man and review its clinical and histological features.
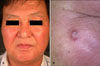
A 63-year-old Korean man presented with a 2-year history of a slowly enlarging, asymptomatic nodule on the right cheek (1). There was no history of loss of weight, loss of appetite, fever, altered bowel habits, rectal bleeding, or melena.
Examination revealed a 1×1 cm, well-demarcated, firm, erythematous nodule on the right cheek. Other skin lesions or lymphadenopathy were not observed. Physical examination was otherwise unremarkable.
A punch biopsy revealed islands of uniform-appearing epithelial cells within abundant pools of mucin, separated by thin fibrovascular septae (Fig. 2). Pools of mucin stained positive for Alcian blue pH 2.5 (Fig. 3A) and PAS (Fig. 3B). These findings were consistent with mucinous carcinoma. The results of immunohistochemical staining showed a positive reaction to synaptophysin, cytokeratin 7, estrogen receptor (ER), and progesterone receptor (PR), and a negative reaction to cytokeratin 20 (Fig. 4).
Chest X-ray, computed tomography, gastrointestinal endoscopy and ultrasounds of the parotid glands, thyroid gland and abdomen failed to detect a primary internal site of involvement.
The patient was transferred to the department of plastic surgery, the lesion removed by wide surgical excision, and a rotation flap performed. There has not been any clinical recurrence of the tumor 18 months after the operation.
Primary mucinous carcinoma of the skin is a rare malignant tumor related to the eccrine secretory coil2,3. Most patients are middle-aged or elderly, with men being affected more often than women. Common sites of involvement are the eyelids, cheeks and scalp. Other less common sites include the axillae, feet, chest wall and vulva.
Primary mucinous carcinoma is thought to arise from sweat glands. However, it is still controversial whether this neoplasm has an eccrine or apocrine origin. In a histochemical study, it was suggested that primary mucinous carcinoma undergoes eccrine differentiation based on findings such as abundant phosphorylase activity, very limited nonspecific esterase and acid phosphatase activities, and strong positive reactions for a number of mitochondrial oxidative enzymes13. Additionally, by electron microscopy, the neoplastic cells of primary mucinous carcinoma of the skin were shown to be similar to the dark cells of normal eccrine glands3,14.
On the other hand, there have been some reports suggesting that mucinous carcinoma has apocrine-type differentiation. A case of mucinous carcinoma showed decapitation secretion by the neoplastic cells15. And a case of mucinous carcinoma showed certain ultrastructural characteristics of neoplastic cells with apocrine differentiation such as electron dense bodies and vacuolization16.
Histologically, mucinous carcinoma is characterized by abundant pools of pale-staining mucin which surround island-like aggregates of basaloid tumor cells4. Thin fibrous septae separate individual collections of mucin, giving the tumor a somewhat lobulated appearance. Pools of mucin stained positive for Alcian blue pH 2.5, PAS. The diagnosis of cutaneous mucinous carcinoma is based on distinctive histological features showing multiple small tumor cell islands floating in pools of mucin.
The results of immunohistochemical staining showed positive reactions to synaptophysin, cytokeratin 7, ER and PR, and a negative reaction to cytokeratin 20. These findings indicate neuroendocrine differentiation of the primary cutaneous mucinous carcinoma and suggest that primary cutaneous mucinous carcinomas may span a morphologic spectrum similar to their mammary counterparts17.
It is interesting to note that the tumor cells in our case stained positive for neuroendocrine markers. A group of human breast carcinomas show histochemical evidence of neuroendocrine differentiation. Neuroendocrine differentiation of mucinous carcinoma of the breast has been reported since 1980, with the tumor being classified as type A (without neuroendocrine differentiation), type B (with neuroendocrine differentiation) and type AB (the intermediate form)18.
The expression of neuroendocrine markers is not unique to mucinous carcinoma of the breast. The criteria for diagnosing neuroendocrine differentiation in breast carcinomas are variable, although most authors have based the diagnosis on immunohistochemistry. Neuroendocrine (NE) cells form a small, intrinsic component of the normal breast epithelium19. NE cells secrete neuropeptides (serotonin, calcitonin and others) and specific NE products (chromogranins, neuron-specific enolase (NSE))19.
The significance of neuroendocrine differentiation in mucinous carcinoma of the breast has not been well established. Two previous studies suggested that mucinous carcinoma of breast showing neuroendocrine differentiation had a good prognosis18,19. And these groups were characterized by older patient age, and were associated with more favorable histologic and immunohistochemical parameters including lower tumor nuclear grade, lower incidence of axillary lymph node metastasis, and lower cerbB2 oncoprotein expression18. In a study regarding mucin-producing sweat gland carcinoma, neuroendocrine differentiation was associated with good prognosis20.
Primary cutaneous mucinous carcinoma with neuroendocrine differentiation is an underrecognized low-grade sweat gland carcinoma. It was first described by Rahilly et al.21 in 1995. Although data are limited, analysis of the reported cases shows that cutaneous mucinous carcinoma with neuroendocrine differentiation is indistinguishable from cutaneous mucinous carcinoma by light microscopy and mucin histochemistry22. ER, PR, and NSE are detected by immunohistochemistry, and positivity for chromogranin and synaptophysin is the norm22. And various markers of endocrine differentiation have been used by different groups22. It is not known what proportion of cutaneous mucinous carcinoma shows neuroendocine differentiation, as such markers are rarely reported in the literature.
Ultrastructural findings of primary cutaneous mucinous carcinoma with neuroendocrine differentiation were reported by Rahilly et al.21 and included dark and pale cells (as seen in primary cutaneous mucinous carcinoma) with the presence of membrane-bound dense core granules.
Zembowicz et al.20 presented a series of 12 cases of cutaneous endocrine mucin-producing sweat gland carcinoma, exhibiting similar clinicopathologic features. They suggested that endocrine mucin-producing sweat gland carcinoma may be a precurcor of invasive mucinous carcinoma. Clinical follow up of their cases indicated a favorable prognosis, but the authors emphasized a need for longer follow up20. They also noted a well-documented metastatic potential for endocrine mucin-producing sweat gland carcinoma.
Only eight other cases5-12 of primary cutaneous mucinous carcinoma have been reported in the Korean dermatological literature (Table 1) and none of these showed neuroendocrine differentiation. However, it is difficult to reach accurate conclusions about neuroendocrine differentiation because neuroendocrine markers have not been studied in the Korean literature.
Differential diagnosis include benign tumors such as epidermoid cyst, neuroma, lacrimal sac tumor, pilomatrixoma, chalazion, hordeolum, hemangioma, pyogenic granuloma, lipoma, papilloma, keratoacanthoma, but also malignant tumors such as sebaceous carcinoma, cystic basal cell carcinoma, squamous cell carcinoma, melanoma, Kaposi's sarcoma, and adenoid cystic carcinoma, as well as metastatic adenocarcinoma23.
Mucinous adenocarcinoma metastatic to the skin may arise from the breast, colon, stomach, pancreas, lung, salivary gland, prostate and ovary. Therefore, a work-up to rule out metastatic lesions is necessary in all patients with cutaneous mucinous carcinoma as metastatic disease has important implications for management and prognosis.
In both primary and metastatic mucinous adenocarcinoma, islands of tumor cells appear to float in the lakes of mucin, but the tumor cells are more atypical in the metastatic type, and the atypical cells invade between collagen bundles at the margin of the nodule. Nevertheless metastatic adenocarcinoma may be histologically indistinguishable from primary cutaneous mucinous carcinoma23.
The histopathological clue to an intestinal origin of mucinous carcinoma is the combination of dirty necrosis and the presence of epithelial cells with absorptive/goblet cell differentiation17. Since most colorectal cancers express CK 20, the absence of CK 20 may also be helpful in excluding this diagnosis17.
Although primary cutaneous mucinous carcinoma is usually an indolent tumor with slow growth, it often shows locally aggressive behavior and recurs locally. The recurrence rate after excision with narrow margins is up to 30~40%, especially for lesions located on the eyelid23,24. Therefore the recommended treatment is a wide surgical excision. Mohs surgery is an alternative method25.
In conclusion, we report the present case to alert dermatologists to the occurrence of primary cutaneous mucinous carcinoma on the face. This carcinoma demonstrates neuroendocrine differentiation similar to that of mammary mucinous carcinoma, indicating a degree of homology between mammary and cutaneous mucinous carcinoma. The possibility of the primary tumor being at another site should be considered to exclude the possibility of metastatic mucinous carcinoma before a diagnosis of primary cutaneous mucinous carcinoma is made.
Mucinous carcinoma needs to be surgically removed with wide excision margins and carefully followed up for signs of recurrence.
Figures and Tables
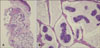 | Fig. 2(A) Well-defined lobulated tumor nodule occupying the lower dermis and subcutaneous tissue (H&E stain, ×40). (B) Islands of uniform-appearing epithelial cells within abundant pools of mucin, separated by thin fibrovascular septae (H&E stain, ×400). |
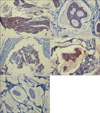 | Fig. 4Results of immunohistochemical staining showed a positive reaction of synaptophysin (A), cytokeratin 7 (B), estrogen receptor (C), and progesterone receptor (D), and a negative reaction of cytokeratin 20 (E) (×400). |
References
1. Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol. 1952. 64:865–880.

2. Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol. 1971. 103:68–78.

3. Wright JD, Font RL. Mucinous sweat gland adenocarcinoma of eyelid: a clinicopathologic study of 21 cases with histochemical and electron microscopic observations. Cancer. 1979. 44:1757–1768.

4. Snow SN, Reizner GT, Dudley CM, Mohs F. Miller SJ, Maloney ME, editors. Eccrine mucinous carcinoma. Cutaneous oncology: pathophysiology, diagnosis, and management. 1998. Oxford: Blackwell Science;758–762.
5. Chang SE, Cheong JK, Choi JH, Sung KJ, Moon KC, Koh JK. Primary mucinous carcinoma of the skin with unusual histopathological features. Ann Dermatol. 1998. 10:266–269.

6. Shin JH, Sung HT, Jung SY, Lee ES. A case of primary cutaneous mucinous adenocarcinoma. Korean J Dermatol. 1999. 37:523–527.
7. Kim MB, Oh CK, Jang HS, Kwon KS. A case of primary mucinous eccrine carcinoma of the skin treated by Mohs micrographic surgery. Korean J Dermatol. 2000. 38:1106–1110.
8. Ku BS, Kwon OE, Kim HS, Kim DC, Song KH, Lee CW, et al. A case of primary mucinous carcinoma of the skin. Korean J Dermatol. 2005. 43:1228–1232.
9. Kim YJ, Kang HY, Lee ES, Kim YC. A case of cutaneous mucinous eccrine carcinoma. Korean J Dermatol. 2006. 44:1098–1101.
10. Lee JS, Yun SJ, Lee JB, Kim SJ, Lee SC, Won YH. A case of primary cutaneous mucinous carcinoma on the chest. Korean J Dermatol. 2008. 46:660–664.
11. Kwon H, Kim DH, Lee J, Park YL, Cho MK, Whang KU. A case of cutaneous mucinous eccrine carcinoma. Korean J Dermatol. 2009. 47:108–110.
12. Hwang JI, Yu DS. A case of mucinous eccrine carcinoma of the skin. Korean J Dermatol. 2009. 47:618–620.
13. Headington JT. Primary mucinous carcinoma of skin: histochemistry and electron microscopy. Cancer. 1977. 39:1055–1063.

14. Yamamoto O, Nakayama K, Asahi M. Sweat gland carcinoma with mucinous and infiltrating duct-like patterns. J Cutan Pathol. 1992. 19:334–339.

15. Saigal RK, Khanna SD, Chander J. Apocrine gland carcinoma in axilla (report of a case). Ind J Dermatol. 1971. 37:177–180.
16. Yeung KY, Stinson JC. Mucinous (adenocystic) carcinoma of sweat glands with widespread metastasis. Case report with ultrastructural study. Cancer. 1977. 39:2556–2562.

17. Kazakov DV, Suster S, LeBoit PE, Calonje E, Bisceglia M, Kutzner H, et al. Mucinous carcinoma of the skin, primary, and secondary: a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol. 2005. 29:764–782.
18. Tse GM, Ma TK, Chu WC, Lam WW, Poon CS, Chan WC. Neuroendocrine differentiation in pure type mammary mucinous carcinoma is associated with favorable histologic and immunohistochemical parameters. Mod Pathol. 2004. 17:568–572.

19. van Krimpen C, Elferink A, Broodman CA, Hop WC, Pronk A, Menke M. The prognostic influence of neuroendocrine differentiation in breast cancer: results of a long-term follow-up study. Breast. 2004. 13:329–333.

20. Zembowicz A, Garcia CF, Tannous ZS, Mihm MC, Koerner F, Pilch BZ. Endocrine mucin-producing sweat gland carcinoma: twelve new cases suggest that it is a precursor of some invasive mucinous carcinomas. Am J Surg Pathol. 2005. 29:1330–1339.
21. Rahilly MA, Beattie GJ, Lessells AM. Mucinous eccrine carcinoma of the vulva with neuroendocrine differentiation. Histopathology. 1995. 27:82–86.

22. Bulliard C, Murali R, Maloof A, Adams S. Endocrine mucin-producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006. 33:812–816.

23. Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol. 2000. 22:166–170.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download