Abstract
Pyoderma gangrenosum (PG) manifests as recurrent deep ulceration of the skin and PG is associated with a variety of disorders. Approximately 30% of the cases of PG develop in patients with inflammatory bowel disease. A 34-year-old woman presented with a one-week history of recurrent ulcers on the right cheek and back. She was diagnosed with ulcerative colitis (UC) 4 years previously and with PG 1 year previously. The clinical course of the skin lesions followed the status of her UC. The patient's skin lesions and bowel symptoms were not improved with prednisolone. After she was started on mesalazine, we observed rapid resolution of skin lesions and bowel symptoms. Herein, we report a case of recurrent PG with UC, and we discuss the possible association between these two conditions, and the efficacy of mesalazine therapy for the treatment of PG combined with UC.
Pyoderma gangrenosum (PG) is a rare, inflammatory, non-infective and non-neoplastic skin disorder. PG is often associated with systemic conditions such as inflammatory bowel disease, rheumatoid arthritis, paraproteinaemia and hematologic malignancies. The precise clinical and pathophysiologic association of PG with an underlying systemic disease is currently unknown. Conventionally, it was thought that PG was not a manifestation or complication of these diseases, and the relationship of PG and combined diseases during their clinical course is not fully understood1. We herein report a patient with recurrent PG, the clinical course of which was correlated with the underlying ulcerative colitis (UC). The patient was treated with mesalazine and her symptoms rapidly resolved.
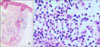
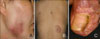
A 34-year-old woman presented with tender, necrotizing ulcers on the right cheek, back and the right great toe for 1 week (Fig. 1). Table 1 summarizes the patient's clinical history. She was previously diagnosed with UC in January 2004 and was treated with systemic corticosteroid and sulfasalazine until October 2006. Ten months after sulfasalazine withdrawal, in August 2007, necrotic ulcers on the right cheek and the back began to develop, with aggravation of her bowel symptoms. At that time, histologic examination of tissue specimens from the right cheek showed perivascular and perifollicular mixed infiltration of neutrophils and lymphocytes from the epidermis to the subcutis (Fig. 2), and no bacterial organism was identified by culture. According to the lesion's histological features, there was evidence of neutrophilic infiltration in the absence of leukocytoclastic vasculitis. With malignancy and infective causes excluded, the skin lesions were diagnosed as PG and she was treated with prednisolone (30 mg/day). With taking oral prednisolone, her abdominal symptoms and skin lesions improved and the steroid therapy stopped in November 2007.
However, at this time, similar skin lesions associated with abdominal pain and bloody diarrhea recurred. Neither bacteria nor fungus was identified from wound culture. She underwent colonoscopy, which revealed severe ulceration with mucosal bleeding in the distal colon. Under the diagnosis of recurrent PG with aggravation of UC, prednisolone (40 mg/day) was recommenced, but her skin lesions and the bowel symptoms deteriorated. Mesalazine therapy was subsequently added to control UC. Her bowel symptoms and skin lesions both gradually improved after 7 days of prednisolone (35 mg/day) and mesalazine (4 g/day) treatment. The prednisolone was tapered over 2 months according to her clinical improvement, and the PG lesions resolved although they did leave some scars (Fig. 3).
Ever since four case reports of PG developed in chronic UC patients have been published in 19302, several reports have suggested that the development of PG was closely associated with active UC or Crohn's disease3-5. In our case, it is remarkable that the development and recurrence of PG paralleled the clinical course of the patient's UC. However, in a study of 86 patients with PG, there was no consistent relationship between PG disease course and that of other associated diseases including UC6. As such, the relationship between the clinical course of PG and UC remains controversial.
To clarify the uncertain relationship between PG and UC, an understanding the pathogenic relationship is important. There are some previous reports suggesting that UC and PG, at least in part, share a common pathogenic immune mechanism. For example, one report proposed that the skin merely reflected the primary pathogenic process in the colon as a Shwartzman phenomenon. Another report suggested that immune complexes from inflamed intestinal mucosa caused cutaneous lesions7,8. Furthermore, recent reports suggest that IL-15 and IL-8 play an important role in the relationship of PG and UC9,10.
In our case, the patient's skin lesions improved after initiating systemic mesalszine therapy. Mesalazine is the standard first line treatment for mild to moderate UC, and is thought be anti-inflammatory through induction of peroxisome proliferator-activated receptor-gamma (PPAR-γ) gene expression and nuclear factor kappa-B (NFκB) activation, as well as inhibiting prostaglandin and interleukin-1 synthesis11. However, no studies have yet been published on PPAR-γ gene expression, NFκB activation or prostaglandin and interleukin-1 synthesis in PG. Furthermore, in consideration of possible association between PG and UC, we cannot exclude the possibility that the improvement of PG was secondary to improvement of the UC. However, we suggest mesalazine play a direct role for the treatment of PG. Although no studies have been published on the effect of systemic mesalazine therapy on PG, a case of successfully treated PG with topical mesalazine cream was reported previously12, and the authors suggested that leukocytes' motility and cytotoxicity were suppressed by mesalazine in PG. The patient's bowel symptom and skin lesion started to improve concomitantly a few days after initiating mesalazine. Therefore, we suggest that systemic mesalazine can be an effective option for the treatment of PG, especially when associated with UC.
Figures and Tables
Fig. 1
Painful, necrotic ulcer surrounded by a violaceous border on the right cheek (A) and the back (B). (C) Painful, necrotic ulcer on the periungual area of the right great toe.

Fig. 2
(A) Skin biopsy specimen showed perivascular and perifollicular infiltration of inflammatory cells from upper dermis to the subcutis (H&E, ×40). (B) Inlfammatory cells were composed of neutrophils and lymphohistiocytes (H&E, ×400).
References
1. Frank CP, Bridget CH. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Pyoderma gangrenosum. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;296–302.

2. Brunsting LA, Goeckerman WH, O'Leary PA. Pyoderma gangrenosum: clinical and experimental observations in five cases occurring in adults. Arch Dermatol Syphilol. 1930. 22:655–680.

3. Sheldon DG, Sawchuk LL, Kozarek RA, Thirlby RC. Twenty cases of peristomal pyoderma gangrenosum: diagnostic implications and management. Arch Surg. 2000. 135:564–568.

4. Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore). 1976. 55:401–412.

5. Menachem Y, Gotsman I. Clinical manifestations of pyoderma gangrenosum associated with inflammatory bowel disease. Isr Med Assoc J. 2004. 6:88–90.

6. Powell FC, Schroeter AL, Su WP, Perry HO. Pyoderma gangrenosum: a review of 86 patients. Q J Med. 1985. 55:173–186.

7. Samitz MH. Cutaneous vasculitis in association with ulcerative colitis. Cutis. 1966. 2:383–387.
8. Tripodi Cutrì F, Salerno R, Lo Schiavo A, Gravina AG, Romano M, Ruocco E. Ulcerative colitis associated with leukocytoclastic vasculitis of the skin. Dig Liver Dis. 2009. 41:e42–e44.
9. Nishiwaki T, Ina K, Goto H, Watanabe O, Tsuzuki T, Furuta R, et al. Possible involvement of the interleukin-15 and interleukin-15 receptor system in a heightened state of lamina propria B cell activation and differentiation in patients with inflammatory bowel disease. J Gastroenterol. 2005. 40:128–136.
10. Oka M. Pyoderma gangrenosum and interleukin 8. Br J Dermatol. 2007. 157:1279–1281.
11. Sandborn WJ. Oral 5-ASA therapy in ulcerative colitis: what are the implications of the new formulations? J Clin Gastroenterol. 2008. 42:338–344.
12. Sanders CJ, Hulsmans RF. Successful treatment of pyoderma gangrenosum with topical 5-aminosalicylic acid. Cutis. 1993. 51:262–264.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download