Abstract
Background
Chemokines and their receptors are important players in tumorigenesis by facilitating tumor proliferation and metastasis. Little is known about the possible function of chemokine receptors in relation to the development and progression of malignant cutaneous tumors.
Objective
The aim of this study was to determine the chemokine receptor CCR3 expression pattern and the protein expression level in selected malignant cutaneous tumors.
Methods
Four types of cell lines (G361, A431, SK-MEL-2, SK-MEL-24) were analyzed, using Western blotting, for the expression of CCR3 protein. Immunohistochemical staining for CCR3 was done on 36 skin cancer tissue samples that included 16 squamous cell carcinomas (SCCs), 16 basal cell carcinomas (BCCs), 16 malignant melanomas (MMs) and 6 normal tissue samples.
Results
Western blot analysis showed that CCR3 protein was more expressed in the MM cell lines (G361, SK-MEL-2,SK-MEL-24) than that in the SCC cell line (A431), and the immunohistochemical analysis showed that CCR3 protein was overexpressed in MM and SCC, it was mildly expressed in BCC and it was hardly expressed in normal tissue.
The incidence of skin cancer is increasing with the increase of the average human life. The domestic reports have also shown this tendency1-4. It has been proven that chronic irritation, inflammation due to infection or chemical carcinogenic factors is related with cancer formation5-7. The internal environment of cancer tissue is controlled by various inflammatory cells, and the acceleration of cell proliferation and halting of cell death by the inflammatory cells, which also promote cell migration, are essential factors in formation and progression of cancer8. There are various intermediate factors common to the inflammatory processes and cancer formation, for example: angiogenic factors, tumor growth factors, organolytic factors and adhesion inducing factors. Of these factors, chemokines and the chemokine receptor system are formed by inflammatory cells and cancer cells, and they induce reactions to each other. Chemokines have also been implicated in cellular transformation, tumor growth and invasion and homing of metastasis to distant sites9. The coordinated secretion of chemokines and their binding to receptors on the cell surface directs leukocyte cell homing to specific tissue sites10,11.
It has recently been revealed that a CCR3/eotaxin-1 loop in T-cell lymphomas is involved in the induction of malignant cell growth12. It has been shown that human renal cell carcinoma's CCR3 and CCR3 expressions are correlated with a higher grade of malignancy of the tumor cells13.
The CCR3 expression in skin tumors such as cutaneous lymphoma has been studied, but it has not been studied at all in other skin tumors as melanoma, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).
The aim of this study was to investigate the expression of CCR3 chemokine receptor in vitro and in vivo. We hypothesized that in malignant skin cancer, there will be a greater CCR3 expression in metastatic skin cancers than that in non metastatic skin cancer. Western blot analysis showed that there was more CCR3 protein detected in the malignant melanoma (MM) cell lines than that in the SCC cell line.
Therefore, we studied the expression of CCR3 in normal skin tissues and malignant skin tumor tissues, including BCC, SCC and MM, to reveal the relationship between CCR3 and skin tumors by using an immunochemical method. This study demonstrated that CCR3 was more expressed, according to immunohistochemistry, in MM, and this was followed by SCC and BCC.
The human MM cell line G361 was obtained from the American Type Culture Collection (CRL 1424; Rockville, MD, USA). The human MM cell line SK-MEL-2 was obtained from the American Type Culture Collection (HTB-68). The human SCC cell line A431 was obtained from the American Type Culture Collection (CRL 1555). The human MM cell line SK-MEL-24 was obtained from the American Type Culture Collection (HTB-71).
The SK-MEL-2 cells and SK-MEL-24 cells were cultured in Minimum Essential Medium supplemented with 10% fetal calf serum and nonessential amino acids. The cells were grown in monolayers in tissue culture flasks that were maintained in a 95% air, 5% CO2 atmosphere at 37℃ in a humidified incubator. The G361 cells and A431 cells were grown in Dulbecco's modified Eagle's medium supplemented with 25 mM HEPES, 100µg/ml penicillin/streptomycin, 4 mM L-glutamine and 10% fetal bovine serum at 37℃ in a 5% CO2 humidified-air incubator.
Specimens were obtained from patients who underwent surgery between January 2002 and July 2009 at the Departments of Dermatology and Plastic and Reconstructive Surgery at Soonchunhyang University Hospital. Content for the biopsy was obtained from the patients before surgery.
Archival formalin-fixed, paraffin-embedded tissues were used for the immunohistochemical studies. The specimens consisted of 16 samples of MM, 16 samples of SCC, 16 samples of BCC and six samples of normal human skin.
The proteins from the four cell lines were separated on NuPAGE 4~12% bis-Tris polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and then the proteins were electrophoretically transferred to immunoblot PVDF membranes. The membranes were then incubated for 1 h at room temperature with a 1:1000 dilution of CCR3/CKR3 (C-term) rabbit monoclonal antibody (Epitomics® Inc., Burlingame, CA, USA). Next, horseradish peroxidase-conjugated secondary antibody (Cell signaling Technologies, Beverly, MA, USA) was applied at a dilution of 1:5000. Anti-beta-actin antibody was used as a loading control and the signal was visualized using an ECL detection kit (Amersham, UK).
For immunohistochemical analysis, paraffin sections (4µm) were prepared and staining was performed by the ABC method. In brief, the slides were deparaffinized in xylene and rehydrated with ethanol. The endogenous peroxidase activity was inhibited by immersing the slides in 3% H2O2/methanol. Antigen retrieval was done with 10mM citrate buffer (pH 6.0) and the slides were heated in a microwave oven for 15 minutes. Pre-incubation with blocking solution for 30 minutes was done to avoid unspecific binding. The section were then incubated overnight at 4℃ with the primary CCR3/CKR3 (C-term) rabbit monoclonal antibody (Epitomics® Inc, 1:100). The slides were consecutively incubated with biotinylated secondary antibody for 30 minutes and then the slides were incubated for 30 minutes with streptavidine-peroxidase. Visualization of the immunoreaction was performed with 3-3'-diaminobenzidine. Negative controls were performed as previously described by substituting PBS for the primary antibody.
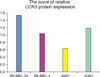
The results of the Western blot analysis showed that CCR3 was expressed in the four cell lines. However, CCR3 protein was more expressed in the MM cell lines than that in the SCC cell line. Significantly, CCR3 was found to be more expressed in the SK-Mel-24 cells (derived from a lymph node metastatic site) than that in both the G361 and SK-Mel-2 cell lines (Fig. 1). The relative abundance of each protein expression was analyzed using Phosphor-Imager software (TINA; Raytest, Straubenhardt, Germany). We measured the score of the CCR3 expression in the MM cell lines and the SCC cell line. There were differences in the TINA scores of CCR3 between the MM cell lines and the SCC cell line. The scores of the relative expression on Western blotting are graphed in Fig. 2.
As shown in Table 1, 16 (100%) of the 16 cases of MM displayed 2+ to 3+ staining intensity for CCR3. Of the 16 labeled cases, 0 (0%) had fewer than 25% of the tumor cells stained, 8 cases (50%) had 25% to 75% of the tumor cells stained and 8 cases (50%) had more than 75% of the tumor cells stained. Sixteen of 16 cases (100%) of the SCC displayed 1+ to 2+ staining intensity for CCR3. Of the 16 labeled cases, 5 (31%) had fewer than 25% of the tumor cells stained, 8 (50%) had 25% to 75% of the tumor cells stained and 3 (19%) had more than 75% of the tumor cells stained. In contrast with MM and SCC, only 4 (25%) of the 16 cases of BCC were immunohistochemically positive for CCR3. The 4 labeled cases each displayed 1+ to 2+ staining in fewer than 25% of the neoplastic cells.
Of the tumor cells, CCR3 was more distinctly expressed in the MM, followed by SCC and BCC on immunohistochemistry (Fig. 3).
Chemokine is a factor that's secreted from damaged or infected tissues for attracting inflammatory cells like leukocytes to specific sites to induce an inflammatory and immunologic reaction by the host. About 50 kinds of chemokines have been reported. It has recently been found that chemokines are also secreted by most cancer cells.
The function of chemokines in cancer cells is complex. Many chemokines show antitumor activity through the activation of immunologic cells or suppression of neovasularization, while other chemokines increase the growth and metastasis of cancer by directly activating cancer growth and increasing cell migration or angiogenesis8.
Studying the expression of chemokine receptors in skin cancers may be especially important due to the increasing incidence of skin cancers. The chemokines exert their biological effects by interacting with G protein-linked transmembrane receptors, called chemokine receptors, that are selectively found on the surfaces of their target cells. There have been about 20 chemokine receptors described11,14-16. There are many reports about cancers being related to CXC chemokine. It is well known that neoangiogenesis is related to the CXC chemokine in non-small cell lung cancer, but the relation of neoangiogenesis to CC chemokine is not known. The role of chemokine receptors in metastasis has only recently been explored.
The chemokine receptors related to melanoma have been revealed to be CXCR4, CCR7, CCR9 and CCR10 through several studies. Müller et al.16 reported CXCR4 plays a role in the migration and progression of MM. Takeuchi et al.17 demonstrated the expression of functional CCR7 in several human melanoma cell lines and in primary and metastatic melanomas and melanoma cell lines under the induction of CCL21/SLC. CCR9 was shown to be associated with intestinal melanoma metastasis18, and CCR10 was shown to be associated with lymph node dissemination19. Murakami et al.20 demonstrated in murine melanoma that B16 cells overexpressing CCR10 resisted the host immune responses and they readily formed tumors. Human primary and metastatic melanomas express CCR10 protein and mRNA.
In SCC, CXCR2 and CCR7 are thought to be tumor associated chemokine receptors. Protection of tumor cells from apoptosis through PI3K activation has been reported after CCR7 activation in head and neck SCC21. CXCR2 is related to metastatic behavior in murine squamous carcinoma cells22.
As mentioned above, eotaxins and CCR3 interaction regulate the Th2-dominant tumor environment, which is closely related to the development of CTCL23. It has been shown that CCR3 is preferentially expressed in vitro by Th-2 cells12. Further, the lesional skin of CTCL at advanced stages contains more eotaxin-3 and CCR3 mRNA as compared with that of early CTCL23, and that study also showed that CCR3+ lymphocytes were present in the lesional skin of CTCL. It is likely that the CCR3+ lymphocytes were induced to migrate into lesional skin by eotaxins, as there was significant correlation between the eotaxin-3 and CCR3 expressions in the lesional skin of CTCL23.
It is known that CCR3 is expressed in eosinophils, basophils and Th2 lymphocytes and these types of cells play a role in allergic inflammation. CCL11/eotaxin and CCL13/MCP-4, which are the ligands of CCR3, attract neutrophils for secreting histamine and this induces allergic inflammation. In addition, CCL5/RANTES, CCL7/MCP-3, CCL8/MCP-2, CCL15/HCC-2, CCL24/Eotaxin-2 and CCL26/Eotaxin-3 have been reported to be the ligands of CCR3. It was also reported that CCR3 is increased in the inflammatory cells that have infiltrated Hodgkin's lymphoma24,25. Jöhrer et al.13 reported that CCR3 is expressed in renal cell carcinoma and they found that the CCR3 expression is correlated with a higher grade of malignancy. In our study, the CCR3 protein expression seen on the immunohistochemical analysis of the samples from the majority of patients was correlated with the degree of malignancy.
We also evaluated the expression of CCR3 protein in MM, SCC and BCC using immunohistochemistry.
In this study, CCR3, which indicates the potentiality of carcinogenesis, was checked in the BCC, SCC and MM using an immunohistologic method. The results show that the expression of CCR3 was weakly positive in 16 cases of BCC with slow growth and poor metastasis. In 16 cases of SCC, which is aggressive and very locally invasive but not metastatic, the expression of CCR3 was weakly positive or moderately positive. In 16 cases of MM, which is invasive and it may metastasize to distant sites through hematogenous and lymphagenous paths, the expression of CCR3 was moderately positive or strongly positive. To the best of our knowledge, the CCR3 expression in cutaneous BCCs, SCCs and MMs has not yet been studied.
We have shown that CCR3 protein is expressed in the G361 melanoma cell line, the A431 SCC cell line, the SK-MEL-2 melanoma cell line and the SK-MEL-24 melanoma cell line. Interestingly, CCR3 protein was more expressed in the MM cell lines than that in the SCC cell line. Significantly, CCR3 was found to be more expressed in the SK-Mel-24 cell line (derived from a lymph node metastatic site) than in both the G361 and SK-Mel-2 cell lines by Western blot analysis.
Immunohistochemical study further supported that the Western blot analysis that CCR3 expression was increased in MM and SCC. The CCR3 protein expression was correlated with a higher grade of malignancy.
We believe that a CCR3 expression might facilitate the proliferative responses in tumor cells and it may be involved in the development and progression of the disease.
Figures and Tables
Fig. 1
CC chemokine receptor CCR3 protein was expressed on squamous cell carcinoma cell line, malignant melanoma cell lines by western blot analysis.
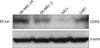
Fig. 3
Representative immunohistochemistry staining for CCR3 protein expression in paraffin-embedded malignant melanoma (A: strongly positive immunostaining, ×200), squamous cell carcinoma (B: moderately positive immunostaining, ×200), basal cell carcinoma (C: weak positive immunostaining, ×200) and normal skin (D: no immunostaining, ×100).
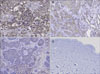
Table 1
Immunohistochemical results for CCR3 protein in malignant melanoma, squamous cell carcinoma, basal cell carcinoma, normal skin
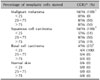
*Expressed as number of positive cases/number of total cases (percentage). †Moderate to strong (2+ to 3+) staining intensity in all positive cases. ‡Weak to moderate (1+ to 2+) staining intensity in all positive cases. §Among the 4 positive cases, 3 had weak (1+) and 1 had moderate (2+) staining intensity.
References
1. Shin JH, Cho SY, Whang KK, Hahm JH. An epidemiologic analysis of cutaneous malignant tumors over 15 years (1984~1998). Korean J Dermatol. 1999. 37:1743–1751.
2. Seo PG, Moon SE, Cho KH. A statistical study of cutaneous malignant tumors (1996~2000). Korean J Dermatol. 2002. 40:129–137.
3. Yoon TJ, Kim HS, Oh CW, Kim TH. A clinical investigation of cutaneous malignant tumors in western Gyeongnam province. Korean J Dermatol. 1997. 35:956–962.
4. Jeong KB, Kim HC, Shin DH, Choi JS, Kim KH. A clinical observation of cutaneous premalignant and malignant tumors. Korean J Dermatol. 2002. 40:924–931.
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. 454:436–444.

7. Mantovani A. Cancer: inflaming metastasis. Nature. 2009. 457:36–37.
9. Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995. 270:27348–27357.

10. Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998. 188:373–386.

11. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000. 12:121–127.
12. Kleinhans M, Tun-Kyi A, Gilliet M, Kadin ME, Dummer R, Burg G, et al. Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T-cell lymphoma. Blood. 2003. 101:1487–1493.

13. Jöhrer K, Zelle-Rieser C, Perathoner A, Moser P, Ramoner R, et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005. 11:2459–2465.

14. Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998. 220:1–17.

15. Schweickart VL, Epp A, Raport CJ, Gray PW. CCR11 is a functional receptor for the monocyte chemoattractant protein family of chemokines. J Biol Chem. 2000. 275:9550–9556.

16. Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001. 410:50–56.

17. Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004. 10:2351–2358.

18. Letsch A, Keilholz U, Schadendorf D, Assfalg G, Asemissen AM, Thiel E, et al. Functional CCR9 expression is associated with small intestinal metastasis. J Invest Dermatol. 2004. 122:685–690.

19. Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, et al. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer. 2006. 42:1181–1187.

20. Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, et al. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med. 2003. 198:1337–1347.

21. Wang J, Seethala RR, Zhang Q, Gooding W, van Waes C, Hasegawa H, et al. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst. 2008. 100:502–512.

22. Loukinova E, Dong G, Enamorado-Ayalya I, Thomas GR, Chen Z, Schreiber H, et al. Growth regulated oncogene-alpha expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC receptor-2 dependent mechanism. Oncogene. 2000. 19:3477–3486.

23. Miyagaki T, Sugaya M, Fujita H, Ohmatsu H, Kakinuma T, Kadono T, et al. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-cell lymphoma. J Invest Dermatol. 2010. 130:2304–2311.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download