Abstract
Atopic dermatitis (AD) is a chronic cutaneous inflammatory disease. Various categories of therapeutic medications are used for treating AD. Omalizumab is a monoclonal anti-IgE antibody that binds to IgE molecules at the high-affinity receptor (FcεRI) binding site. Therefore, omalizumab can be used as a potential new systemic treatment agent for recalcitrant AD patients with elevated IgE levels. A 34-year-old man had been treated for AD with several topical and oral agents. However, he was refractory to these therapies and his serum IgE levels were very high. We treated him with omalizumab. After 8 months of the treatment, his symptoms were notably improved and the SCORAD index was decreased. Thus, we report on the first case of recalcitrant AD that was successfully treated with omalizumab in Korea.
Atopic dermatitis (AD) is treated by topical agents, including bland emollients, topical steroids, tacrolimus and pimecrolimus, and also oral agents such as antibiotics, systemic corticosteroids, antihistamines and cyclosporine in proportion to the clinical severity of AD1. However, severe or recalcitrant AD does not often respond satisfactorily to several conventional therapies. Therefore, many dermatologists have tried treating severe or recalcitrant AD using novel modalities such as dapsone2, azathioprine3, mycophenolate mofetil4 and intravenous immunoglobulin5. Omalizumab has recently been used as a potential new systemic treatment for recalcitrant AD patients with elevated IgE levels, and this based on omalizumab's efficacy for treating asthma and allergic rhinitis, which are parts of the classic allergic triad6. Here we report on the first case of the adult AD that was successfully treated with omalizumab in Korea.
A 34-year-old man with over a 30-year history of AD had been treated by several therapies such as topical agents, oral agents, immunotherapy and herb medicine for many years with minimal response. He had also been hospitalized several times to treat his eczema herpeticum or secondary infection.
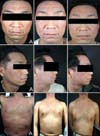
The physical examination revealed erythematous papules, plaques and vesicles with severe lichenification and excoriation over the whole body, and especially on the face and anterior chest. The lesion covered approximately 60% of the total body surface area (Fig. 1A). There was a family history of atopy. He also had asthma, allergic rhinitis and cataract due to long standing conjunctivitis.
Laboratory analyses demonstrated that the serum IgE level was 9,360 IU/ml (normal range: 0~20 IU/ml), the eosinophil cationic protein (ECP) level was above 200 ug/L (normal range: 2~18 ug/L) and the eosinophil portion of the white blood cells was 21.2%. The other laboratory findings, including the hemoglobin and red blood cell count, were within the normal range.
To exclude the possibility of parasitic infection because of the eosinophilia, he took antihelminthics. AD treatment started with cyclosporine (250 mg per day) and hydroxyzine (60 mg per day) with topical steroids and pimecrolimus. Nevertheless, he was refractory to these remedies and vigorously wanted a new treatment regardless of the cost. Thus, we decided to try to treat the patient with omalizumab.
Before the treatment with omalizumab, the patient's SCORAD index was 48. The pruritus scale and insomnia scale were 4 points each, respectively. Omalizumab was administrated subcutaneously at 600 mg every other week for 2 months, and then the dose was decreased to 300 mg over the next 6 months in 2 week intervals. During administration of omalizumab, the oral hydroxyzine and topical treatments were maintained. Our patient didn't suffer from any adverse effects such as an immediate reaction at the injection site and there was a notable improvement of the skin lesions (Fig. 1B). The SCORAD index was decreased from 48 to 35 (Fig. 2A) and the subjective scale for pruritus and insomnia was also greatly decreased (4 to 2 and 4 to 1, respectively) (Fig. 2B, C). He maintained a good state for 6 months. The oral hydroxyzine and topical treatments were maintained after cessation of the omalizumab treatment (Fig. 1C).
There are several clues that IgE is related with AD. AD is associated with elevated levels of total and exoallergen specific serum IgE1. In addition, IgE antibodies against self-proteins such as epithelial extracts are found in the serum specimens from patients with severe AD and the serum levels of IgE autoantibody are correlated with the disease severity7. High-affinity IgE receptor is predominant in AD8.
Omalizumab is a novel therapeutic recombinant DNA-derived humanized IgG16-kappa monoclonal antibody that targets human IgE9. It is currently used for the treating asthma patients who are at least 12 years old and who have serum IgE levels that do not exceed 700 IU/ml9.
The mechanisms of action of omalizumab are summarized as follows. First, omalizumab binds to the Fc region of all forms of circulating IgE and this prevents IgE-medicated reactions10. Second, omalizumab downregulates high-affinity IgE receptor by binding to and inactivating IgE10.
Based on the above mechanism, we tried treating our patient with omalizumab because an elevated serum IgE level of 9,360 IU/ml was noted on the laboratory findings. Our patient's symptoms did not improve for the first 2 months of omalizumab treatment. That can be explained that although the free IgE is extensively and very rapidly suppressed after administrating omalizumab, it takes up to 3 months before the clinical symptoms achieve their new equilibrium values11. In our case, the AD was improved from the third month after the initiation of omalizumab treatment.
Our patient was treated successfully with omalizumab. Some dermatologists have been tried to treat AD patients with omalizumab, and the majority of them were successful in treating AD with omalizumab9,12. On the other hand, a few AD patients failed when being treated with omalizumab6. The reason for the difference between the successful cases and unsuccessful cases may be insufficient dosing of omalizumab6. In the unsuccessful cases, although the patients all are adults, omalizumab was administrated at 450 mg subcutaneously every other week for 4 months6. However, for the successful cases, most of the patients were children and the dosing was similar to that of the patients of the unsuccessful cases9,12. Additionally, the period of administration of omalizumab in the successful cases was longer than that in the unsuccessful cases6,9,12. As mentioned above, it takes 3 months to see an effect with omalizumab. In a similar context, the mean serum IgE levels of the unsuccessful patients were much higher than those in the successful patients6,9,12. If the dose of omalizumab is insufficient, then the drug does not thoroughly block all the IgE. In our case, our patient might have been treated successfully because of the sufficient dosing and dosing period.
In one study, the safety and tolerability of omalizumab were evaluated in 7,500 adult patients13. The results of that study revealed that the adverse effects were similar to that of the placebo and there was no evidence of an increased risk of drug-related hypersensitivity reactions or immune-complex disease. No adverse effects were reported in the other trials of treating AD with omalizumab9,12. Our patient visited every 2 weeks for monitoring the clinical effects and possible adverse effects. Any immediate reaction at the injection site and other adverse effects were not found in our patient.
We report here on the first case of adult AD that was successfully treated with omalizumab in Korea. We suggest that those patients who have AD with a high level of IgE and who do not show any improvement in spite of traditional therapy, including systemic cyclosporine, will be good candidates to try omalizumab as a safe alternative modality.
Figures and Tables
References
1. Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006. 118:152–169.

2. Lee BH, Park CK, Kim HO, Park CW, Lee CH. A case of recalcitrant atopic dermatitis treated with dapsone. Korean J Dermatol. 2005. 43:1626–1630.
3. Kuanprasert N, Herbert O, Barnetson RS. Clinical improvement and significant reduction of total serum IgE in patients suffering from severe atopic dermatitis treated with oral azathioprine. Australas J Dermatol. 2002. 43:125–127.

4. Neuber K, Schwartz I, Itschert G, Dieck AT. Treatment of atopic eczema with oral mycophenolate mofetil. Br J Dermatol. 2000. 143:385–391.

5. Jolles S, Hughes J, Rustin M. The treatment of atopic dermatitis with adjunctive high-dose intravenous immunoglobulin: a report of three patients and review of the literature. Br J Dermatol. 2000. 142:551–554.

6. Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005. 53:338–340.

8. Stingl G. IgE-mediated, Fc(epsilon)RI-dependent allergen presentation: a pathogenetic factor in atopic dermatitis? J Am Acad Dermatol. 2001. 45:S17–S20.
9. Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006. 54:68–72.

10. Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol. 2009. 68:61–76.

11. Salvin RG, Ferioli C, Tannenbaum SJ, Mrtin C, Blogg M, Lowe PJ. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentration. J Allergy Clin Immunol. 2009. 123:107–113.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download