Abstract
Primary inoculation tuberculosis results from the direct inoculation of Mycobacterium tuberculosis into the skin of a person who has no natural or artificially acquired immunity to the organism. The pathogenesis requires a break in the skin from an abrasion or injury that allows entry of the tubercle bacilli. We report 3 cases of primary inoculation tuberculosis resulting from illegal acupuncture. Three patients over 70 years old presented with erythematous, ulcerative, indurated plaques on the back. Skin lesions had developed at the acupuncture sites 1 or 2 weeks after a session of acupuncture, which was intended to relieve back pain. An unlicensed, non-medically trained person conducted each session. The patients' past medical and family histories were unremarkable. Granulomatous inflammatory infiltration and acid-fast bacilli were observed histologically. M. tuberculosis was identified by mycobacterial culture and polymerase chain reaction. Nine months after the initiation of antituberculosis medication, skin lesions improved, and no evidence of recurrence or other organ involvement was observed at the 1-year follow-up visit.
Primary inoculation tuberculosis results from the direct introduction of Mycobacterium tuberculosis into the skin of a person with no previous tuberculosis infection1. Mycobacterium cannot penetrate intact skin, so the pathogenesis for cutaneous inoculation tuberculosis requires a breach of the normal barrier function of the skin from a minor abrasion or injury, allowing entry of the tubercle bacilli2.
Primary inoculation tuberculosis has been reported after vaccination with bacillus Calmette-Guérin (BCG)3, intralesional steroid injection4, needle stick injury5 and blepharoplasty6. Recent case reports have described mycobacterial infection as a complication of acupuncture7,8. Herein, we report 3 cases of primary inoculation tuberculosis resulting from illegal acupuncture in the same nursing home on the same day by a person with no medical training or license.
A 77-year-old male developed multiple erythematous plaques on his back and abdomen 2 weeks after receiving illegal acupuncture by a person with no medical training or license in a nursing home. Two weeks after the symptoms first appeared, he visited our clinic on September 27, 2007. His past medical history and family history were unremarkable. Physical examination revealed indurated, erythematous plaques on the back and right lower abdomen without adjacent lymphadenopathy (Fig. 1A). Laboratory tests, including complete blood cell count, blood chemistry studies, urinalysis and chest X-ray, were all normal. A skin biopsy of the abdomen showed diffuse dermal infiltration composed of lympho-histiocytes and abundant neutrophils with necrosis of dermal collagen fibers. Focal aggregation of numerous acid-fast bacilli was identified by Ziehl-Neelsen stain (Fig. 2A). Polymerase chain reaction (PCR) for M. tuberculosis in a formalin-fixed, paraffin-embedded skin biopsy specimen was positive (Fig. 3), but a culture showed no growth. Periodic acid-Schiff stain (PAS stain), non-tuberculosis PCR, and fungal and bacterial cultures were performed for differential diagnosis, and all showed negative results. The results of the laboratory tests of this case and those of the 2 other patients are summarized in Table 1.
A diagnosis of primary inoculation tuberculosis was made, and the patient began a 9-month course of antituberculosis medication, including isoniazid (400 mg/day), rifampicin (600 mg/day), and ethambutol (800 mg/day). At 1 year after treatment, no evidence of recurrence or other organ involvement was seen (Fig. 1B).
On October 8, 2007, a 72-year-old female visited our clinic with a 4-week history of multiple erythematous plaques on her back, shoulder, and right thigh. Skin lesions appeared 10 days after receiving illegal acupuncture by the same person in case 1. The patient had neither a history of tuberculosis infection nor any other health problems. Physical examination revealed indurated, erythematous plaques with purulent discharge on the back, shoulder, and right thigh, without adjacent lymphadenopathy (Fig. 1C, D). Laboratory tests, including complete blood cell count, blood chemistry studies, urinalysis and chest X-ray, were all normal. A skin biopsy of the right thigh showed a deep dermal abscess with inflammatory cellular infiltration and foreign body reaction (Fig. 2B). M. tuberculosis grew from an 8-week culture of the pus (Fig. 4), and PCR of the colony for M. tuberculosis showed a positive result. After 9 months of antituberculosis medication, skin lesions had resolved with mild hyperpigmentation.
A 75-year-old female visited our clinic on October 24, 2007 complaining of a 6-week history of multiple erythematous plaques on her back and both thighs, which appeared 1 week after receiving illegal acupuncture by the same person in cases 1 and 2. She was treated with topical antibiotics and traditional medicines for 1 month without success. She had neither a history of tuberculosis infection nor other health problems. Physical examination revealed indurated, erythematous plaques and ulcerations with a purulent pseudomembrane on the back and both thighs without adjacent lymphadenopathy (Fig. 1E). Laboratory tests and chest X-rays were normal. The results of a skin biopsy of the right thigh were similar to those in case 2 (Fig. 2C). After 9 months of antituberculosis medication, the skin lesions had resolved (Fig. 1F).
Cutaneous tuberculosis infection is rare, accounting for 0.1% of all cases seen in a dermatology service9. Clinical manifestation of cutaneous tuberculosis is so variable that a high index of suspicion is required for its diagnosis. Cutaneous tuberculosis can be classified according to the source of skin infection. Endogenous infection occurs in a host previously infected with M. tuberculosis by contiguous hematogenous or lymphatic extension10. Primary inoculation tuberculosis is an exogenous infection, which is caused by the direct inoculation of bacteria into the skin of a person who has no natural or artificially acquired immunity to the organism. It usually occurs through injury to the skin by unnoticed minor trauma in an exposed area11. Our 3 cases developed after illegal acupuncture by an unlicensed, non-medically trained person in a nursing home. In these cases, the exogenous source of infection was probably the needle used during an acupuncture procedure, because every site receiving that procedure developed similar skin lesions. The lesions were found to be compatible with primary inoculation tuberculosis by clinical and histopathologic findings.
In general, inoculation tuberculosis skin lesions appear 2~4 weeks after inoculation and present as an erythematous nonpainful papule or nodule. Histopathology is an acute non-specific inflammatory reaction in both skin and lymph nodes, and the mycobacteria are easily detected by acid-fast bacilli stain. Through lymphatic drainage, painless regional lymphadenopathy develops 3 to 8 weeks after the infection, and a tuberculoid appearance and caseation necrosis may be seen in histopathology1. The multibacillary condition becomes paucibacillary as host immunity develops, and the tuberculin skin test converts to positive. Mycobacteria are difficult to identify by acid-fast bacilli stain at this time11. The patient in case 1 showed a non-specific acute inflammatory reaction by histopathology, and numerous acid-fast bacilli were identified by Ziehl-Neelsen stain, because a skin biopsy was performed 4 weeks after inoculation. In contrast, the patients in cases 2 and 3 showed a deep dermal abscess with inflammatory cellular infiltration and foreign body reaction by histopathology and were negative for acid-fast bacilli by Ziehl-Neelsen stain. The skin biopsies in these cases were performed 5 weeks (case 2) and 7 weeks (case 3) after infection.
Differential diagnoses include infection with atypical mycobacteria (called mycobacteria other than M. tuberculosis or MOTT), sarcoidosis, foreign body granuloma, syphilis or sporotrichosis2. Sarcoidosis and foreign body granuloma do not present with caseation granuloma by histology. Syphilis can be excluded by a serological test, and it shows vascular changes and predominant plasma cell infiltration by histology. PAS stain and fungal culture can be used in the differential diagnosis of sporotrichosis. In general, MOTT are thought to cause mycobacterial skin disease more often than M. tuberculosis. Due to the fact that MOTT infections often closely mimic infections with M. tuberculosis, they are hard to distinguish from M. tuberculosis by clinical manifestation, acid-fast bacilli stain or histopathology11. Diagnosis can be made by identifying the organism by microbiological culture. Many of the atypical mycobacterial organisms grow on routine bacterial culture within 2 to 3 weeks12, whereas M. tuberculosis requires incubation for 6 to 8 weeks, so the difference in the incubation period can be a clue for diagnosis. PCR can also be used in the diagnosis of tuberculosis. The detection of M. tuberculosis by PCR from skin samples is variable, with a sensitivity of about 60~80% and specificity of 100%13. The PCR assay for mycobacterium may be helpful when the results of mycobacterial culture and histopathology are negative.
In the immunocompetent patient, the primary lesion heals with scarring within 1~3 months, but it can be delayed up to 12 months in immunocompromised patients14. In general, the treatment of cutaneous tuberculosis should not differ from tuberculosis of other organs. Multiple antituberculous medications (including isoniazid and rifampin) should be used in combination for a period of no less than 6 months11.
In Korea, the incidence rate of tuberculosis has shown a gradual decline; however, the prevention of inoculation tuberculosis must be encouraged, because many people are unaware of the risk of acquiring tuberculous disease through this possible route. Thorough evaluation and investigation of any suspicious skin lesions are also important.
Figures and Tables
Fig. 1
(A) Indurated, erythematous plaque on back in case 1. (B) The skin lesions improved after treatment with antituberculosis medication for 9 months in case 1. (C, D) Erythematous plaques with purulent discharge on shoulder and back in case 2. (E) Erythematous plaques and ulcers with purulent pseudomembrane on lower back in case 3. (F) After 9 months of treatment, the skin lesions improved in case 3.

Fig. 2
(A) Diffuse dermal infiltration composed of lympho histiocytes and abundant neutrophils with necrosis of dermal collagen fibers and focal aggregation of numerous acid-fast bacilli identified by Ziehl-Neelsen stain (inset) in case 1 (H&E stain, ×100, Inset: Ziehl-Neelsen stain, ×400). (B, C) Deep dermal abscess with inflammatory cellular infiltration and foreign body reaction in case 2 and case 3, respectively (H&E stain, ×100).

Fig. 3
Polymerase chain reaction for Mycobacterium tuberculosis in formalin-fixed, paraffin-embedded skin biopsy specimen showed a positive band of 304 bp in case 1 (M: molecular weight marker, P: positive control, 1, 2, 3: cases 1, 2, 3, N: negative control).
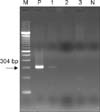
Table 1
Summary of laboratory tests

*Case 1 showed a positive result of PCR for Mycobacterium tuberculosis in both biopsy material and formalin-fixed, paraffin-embedded skin biopsy specimen. In case 2, PCR of the colony for M. tuberculosis, not the formalin-fixed, paraffin-embedded skin biopsy specimen, showed a positive result. TB: tuberculosis bacilli, PCR: polymerase chain reaction, NTM: non-tuberculosis mycobacteria, PAS: periodic acid-Schiff, +: positive, -: negative.
References
1. Kim SW, Kim HU, Lee CJ. Korean Dermatological Association. Tuberculosis of the skin. Dermatology. 2001. 4th ed. Seoul: Yeomungak;292–293.
2. Wong HW, Tay YK, Sim CS. Papular eruption on a tattoo: a case of primary inoculation tuberculosis. Australas J Dermatol. 2005. 46:84–87.

3. Lew W, Kim SM, Lee KH. Unusual skin tuberculosis following BCG vaccination. Korean J Dermatol. 1990. 28:349–352.
4. Kim JC, Park YM, Choi JS, Kim KH. A case of primary inoculation tuberculosis developed after intralesional injection of corticosteroid. Korean J Dermatol. 1991. 29:827–831.
5. You DO, Youn NH, Park SD. A case of primary inoculation tuberculosis. Korean J Dermatol. 2002. 40:1139–1141.
6. Kim MG, Kim JA, Kim WS, Lee DY, Lee ES. A case of primary inoculation tuberculosis. Korean J Dermatol. 2006. 44:94–96.
7. Woo PC, Lau SK, Wong SS, Yuen KY. Staphylococcus aureus subcutaneous abscess complicating acupuncture: need for implementation of proper infection control guidelines. New Microbiol. 2003. 26:169–174.
8. Woo PC, Leung KW, Wong SS, Chong KT, Cheung EY, Yuen KY. Relatively alcohol-resistant mycobacteria are emerging pathogens in patients receiving acupuncture treatment. J Clin Microbiol. 2002. 40:1219–1224.

9. Ho CK, Ho MH, Chong LY. Cutaneous tuberculosis in Hong Kong: an update. Hong Kong Med J. 2006. 12:272–277.
10. Tapias L, Tapias-Vargas LF, Tapias-Vargas L. Primary cutaneous inoculation tuberculosis in a healthcare worker as a result of a surgical accident. Int J Dermatol. 2008. 47:833–835.

11. Tappeiner G. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Tuberculosis and infection with atypical mycobacteria. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1768–1778.
12. Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009. 27:63–73.

13. Cheng VC, Yew WW, Yuen KY. Molecular diagnostics in tuberculosis. Eur J Clin Microbiol Infect Dis. 2005. 24:711–720.

14. Gawkrodger DJ. Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Mycobacterial infections. Rook/Wilkinson/Ebling textbook of dermatology. 1998. 6th ed. Oxford: Blackwell Science;1181–1214.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download