Abstract
Clear cell acanthoma (CCA) is a rare benign epidermal tumor. It usually presents as a flat nodule or dome-shaped plaque and is often localized on the legs of the elderly. We observed an unusual case of polypoid CCA on the nipple of a 14-year-old girl. At present, a few cases of CCA on the nipple area have been reported in the literature. However, CCA presented as a polypoid tumor on the nipple area has been reported very rarely. We herein report the very rare case of polypoid CCA on the nipple and suggest that CCA should be included in the clinical differential diagnosis of polypoid lesions on the nipple.
Clear cell acanthoma (CCA), first described by Degos in 1962, is a rare benign epidermal tumor that is clinically and histologically distinctive1. It usually presents as a solitary, erythematous to brown, well-demarcated papule or plaque with scales2. The legs of the elderly are the most frequently involved sites.
Histopathologically, CCA is typified by a significantly acanthotic epidermis with clear and glycogen-containing epidermal cells that are strongly positive to periodic acid-Schiff (PAS) stain. The epidermis is often psoriasiform, and epidermal ridges are commonly fused. Common features include parakeratosis and infiltration of the epidermis by neutrophils3.
At present, a few cases of CCA on the nipple area have been reported in the literature4-6. Unlike typical CCA, CCA on the nipple area usually presents as chronic eczema rather than as a papule or plaque6. However, CCA as a polypoid tumor on the nipple area has very rarely been reported. We herein report the rare case of polypoid CCA on the nipple and suggest that CCA should be included in the clinical differential diagnosis of polypoid lesions on the nipple.
A 14-year-old girl presented with 1 year of a solitary brownish polypoid nodule on the left nipple. The lesion had first appeared as a small papule and had been growing slowly over time. Symptoms included itching and discharge from the nodule. She had atopic dermatitis, so the nipple lesion was treated as such without any clinical improvement at an outside clinic.
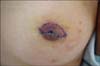
Her family history and past medical history were unremarkable. The skin lesion was a 3.0×1.5×1.3 cm brown polypoid nodule on the left nipple (Fig. 1). Laboratory tests, including blood cell count and blood chemistry, were all within normal ranges. Her IgE serum level, however, was not examined.
Under the presumption of eczema or a benign tumor, a 3 mm punch biopsy of the polypoid nodule was done. Histopathological findings showed acanthosis composed of clear cells, regular elongation of rete ridges and exocytosis of neutrophils in the epidermis.
Based on clinical and histological findings, an initial diagnosis of CCA was made. The patient was referred to the department of plastic surgery for complete surgical excision. Excision was performed without any significant complications. Histopathological findings on the excised specimen were consistent with CCA (Fig. 2). In addition, clear cells stained positively to PAS (Fig. 3). No evidence of recurrence has been observed for 9 months (Fig. 4).
The etiopathogenesis of CCA is not fully understood. The current hypothesis is divided into 2 main theories. The first is that CCA is a kind of benign epidermal neoplasm and the other theory, which argues that CCA is not a true neoplasia, suggests instead that it is a reactive inflammatory dermatoses. In the first concept, the origin of CCA tumor cells is not yet clarified. Jones and Wells7 first reported that CCA is derived from sweat glands. However, Penneys et al.8 reported that carcinoembryonic antigen CEA present in acrosyringium was negative in 2 cases of CCA. Some authors proposed that CCA might be a variant of seborrheic keratosis9. However, Cotton et al.10 found that epithelial membrane antigen (EMA), usually not expressed in seborrheic keratosis, was positive in 5 CCA cases. Previous reports supporting the second concept, which assumes CCA is an inflammatory dermatoses, are as follows. Degos and Civatte11 reported that many CCA cases had concurrent inflammatory skin diseases such as psoriasis and suggested that inflammation might have some role in the development of CCA.
Supporting the inflammatory hypothesis, chronic inflammatory dermatoses, including atopic dermatitis, were found on the nipple area in almost all of the reported cases (Table 1)4,5. Moreover, CCA of these patients manifested as eczematous lesions, not as tumorous conditions4-6. Our patient also had atopic dermatitis and had eczematous lesions on the nipple. However, she had a distinct polypoid nodule, which was diagnosed as a CCA. All patients in Table 1 were Korean. CCA on the nipple area has not yet been found in Caucasians. In Korea, nipple eczema is one of the minor diagnostic criteria of atopic dermatitis. Park et al.12 reported that the incidence of nipple eczema was 6.2% in Korean atopic dermatitis patients above 12 years old. However, the incidence of nipple eczema is much lower in Western atopic dermatitis patients13. This may explain why no reports of CCA on the nipple area are from Western countries.
The majority of patients with CCA are most commonly between the ages of 50 and 70 years, and there is no known sex predilection10. The lesions of CCA usually occur as solitary papules, nodules or small plaques with distinct margins, are asymptomatic, and are often localized on the lower extremities2,3,10. There have been several rare, reported variant forms of CCA, such as giant, multiple, cystic, pigmented, and polypoid, and those associated with melanocytic nevus and epidermal nevus14. Among several variant forms of CCA, 6 cases of polypoid CCA have been found in the literature up to date3,14-18. Polypoid CCA has been found at various body sites, including the scalp, thigh, scrotum and neck. However, we encountered a very rare case of polypoid CCA on the nipple area. The clinical differential diagnosis of a polypoid papule on the nipple includes seborrheic keratosis, histiocytoma, viral wart, nevus and basal cell carcinoma3. However, CCA can be easily differentiated from these conditions by typical histological features.
We herein report the very rare case of polypoid CCA on the nipple and suggest that CCA should be considered in the clinical differential diagnosis of polypoid lesions on the nipple.
Figures and Tables
 | Fig. 2(A) Acanthosis composed of clear cells, regular elongation of rete ridges in the epidermis (H&E stain, ×40). (B) A relative sharp line is drawn between the lesion cells and normal epidermis and the epidermal surface showing parakeratotic scales (H&E stain, ×100). Inlet: Close-up view (H&E stain, ×200). (C) Exocytosis of neutrophils and intradermal neutrophil infiltration are observed (H&E stain, ×400). |
References
1. Degos R, Delort J, Civatte J, Poiares Baptista A. Epidermal tumor with an unusual appearance: clear cell acanthoma. Ann Dermatol Syphiligr (Paris). 1962. 89:361–371.
2. Kim HD, Park SJ, Park YL, Cho MK, Whang KU, Shin EA. A case of clear cell acanthoma on the dorsal foot. Korean J Dermatol. 2006. 44:858–860.
3. Inalöz HS, Patel G, Knight AG. Polypoid clear cell acanthoma: case report. J Eur Acad Dermatol Venereol. 2000. 14:511–512.

4. Kim DH, Kim CW, Kang SJ, Kim TY. A case of clear cell acanthoma presenting as nipple eczema. Br J Dermatol. 1999. 141:950–951.

5. Um SH, Oh CW. Three cases of clear cell acanthoma on nipple and areola. Korean J Dermatol. 2003. 41:85–88.
6. Kim SY, Choi HY, Myung KB, Choi YW. The expressions of cytokeratin 16, involucrin and PCNA in clear cell acanthoma on areola. Korean J Dermatol. 2007. 45:804–810.
7. Jones EW, Wells GC. Degos' acanthoma (acanthome à cellules claires). A clinical and histological report of nine cases. Arch Dermatol. 1966. 94:286–294.

8. Penneys NS, Nadji M, Zeigels-Weissman J. Clear cell acanthoma: not of sweat gland origin. Acta Derm Venereol. 1981. 61:569–570.
9. Kwittken J. Clear cell acanthoma: a metabolic variant of seborrheic keratosis. Mt Sinai J Med. 1980. 47:49–51.
10. Cotton DW, Mills PM, Stephenson TJ, Underwood JC. On the nature of clear cell acanthomas. Br J Dermatol. 1987. 117:569–574.

11. Degos R, Civatte J. Clear-cell acanthoma. Experience of 8 years. Br J Dermatol. 1970. 80:248–254.

12. Park YM, Park HJ, Kim TY, Kim CW. The study on the hospital-base relative frequency, and clinical and laboratory findings of atopic dermatitis. Korean J Dermatol. 1997. 35:96–106.
13. Girolomoni G, Abeni D, Masini C, Sera F, Ayala F, Belloni-Fortina A, et al. The epidemiology of atopic dermatitis in Italian schoolchildren. Allergy. 2003. 58:420–425.

14. Kawaguchi H, Tatewaki S, Takeuchi M. A case of polypoid clear cell acanthoma on the scrotum. J Dermatol. 2004. 31:236–238.

15. Wilde JL, Meffert JJ, McCollough ML. Polypoid clear cell acanthoma of the scalp. Cutis. 2001. 67:149–151.
16. Nijssen A, Laeijendecker R, Heinhuis RJ, Dekker SK. Polypoid clear cell acanthoma of unusual size. J Am Acad Dermatol. 2001. 44:314–316.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download