Abstract
Tubular apocrine adenoma (TAA) is a very rare sweat gland tumor. TAA and syringocystadenoma papilliferum (SCAP) rarely develop together in a nevus sebaceus (NS). Herein, we report on a 40-year-old Korean woman with TAA associated with SCAP that developed in a NS located on the scalp.
Tubular apocrine adenoma (TAA) is a very rare sweat gland tumor that was first described in 19721. Fischer2 considered TAA to be a variant of syringocystadenoma papilliferum (SCAP). However, others have suggested that TAA is an independent clinical entity consisting of a benign appendage tumor of an apocrine origin, and it is often associated with nevus sebaceus (NS)3. We report here on a case of TAA associated with SCAP that developed in a NS on the scalp.
A 40-year-old woman presented with an asymptomatic nodule on the scalp. The lesion first was recognized during childhood as a small plaque that was diagnosed as a NS; the lesion had become elevated as the patient grew. The personal and family histories were not remarkable. Dermatological examination revealed a 2.5 cm diameter, non-tender pedunculated nodule. The surface was erythematous and lobulated (Fig. 1). The tumor was completely excised. The histopathological findings showed 2 distinctly different zones (Fig. 2). The upper portion of the tumor showed cystic and irregularly dilated tubular structures with deep invaginations, from which emerged thick papillomatous projections lined with 2 rows of epithelial cells. The peripheral layer consisted of cuboidal or flattened cells and the luminal layer was composed of columnar cells, and some of which showed decapitation secretion. Numerous plasma cells densely and diffusely infiltrated the stroma (Fig. 3). The lower portion of the lesion consisted of variable sized tubular structures with 2 or more layers of epithelial cells. The outer layer was mostly composed of flattened cells, whereas the inner layer was composed of columnar cells, which showed decapitation secretion. Amorphous material that contained cellular fragments was seen in some of the lumina (Fig. 4). The findings in the upper portion of the lesion were thought to be representative of SCAP and those in the deeper portion were TAA.
Immunohistochemical studies demonstrated that the luminal layer reacted with antiepithelial membrane antigen antibodies and CAM5.2 in both the SCAP and TAA sections. There was strong staining for gross cystic disease fluid protein-15 (GCDFP-15) in the luminal cells of the TAA, but such staining was negative in the luminal cells of the SCAP (Fig. 5). Staining for smooth muscle actin was strong in the peripheral layer of the TAA, but this staining was negative in the SCAP.
The original description of TAA was reported in 19721. Some authors have suggested that TAA represented a variant of SCAP, while Umbert and Winkelmann3 attempted to further differentiate TAA from SCAP. TAA differs from SCAP in several aspects. TAA shows no cystically dilated apocrine invaginations extending down from the epidermis, papillary projections are absent and the stroma of TAA is composed of dense fibrous connective tissue4. Our case showed the characteristic features of SCAP in the upper portion of the tumor and those of TAA in the lower portion of the lesion. Only 7 cases of TAA associated SCAP have been reported in the medical literature, and these cases are summarized in Table 14-10.
An increased incidence of benign and malignant adnexal tumors has been noted to occur within NS. Cribier et al.11 reported that SCAP is the most common benign secondary tumor associated with NS. This is probably due to the presence of ectopic apocrine glands that are located in the deepest part of the NS, which can lead to either apocrine cysts or to SCAP. Ishiko et al.6 suggested that TAA and SCAP occurred together only when they were preceded by an organoid nevus. Five cases of TAA and SCAP were reported to be associated with NS4-6,8,10. However, Lee et al.9 reported 1 case in the external auditory canal that had no evidence of pre-existing NS.
In our case, immunohistochemical studies demonstrated that the luminal layer reacted with antiepithelial membrane antigen antibodies and CAM5.2. Histopathologically, the portion of the inner layer showed active decapitation secretion in both the TAA and SACP lesions. These findings suggest that both tumors may originate from apocrine glands in the deeper portion of the NS. However, the immunohistochemical studies in our case were positive for GCDFP-15 in the TAA lesion, but they were negative in the SCAP lesion. These findings further support the biological differences between the TAA and SCAP apocrine-differentiated tumor, as Yamane et al.10 suggested.
The histogenesis of the SCAP is unclear. While some investigators have suggested that SCAP originates from eccrine elements, and others have suggested that these tumors originate from apocrine elements. Recent studies have suggested that these tumors arise from either pleuripotential appendageal cells or apo-eccrine glands7. Apocrine differentiation has been demonstrated in TAA, but both eccrine and apocrine differentiation have also been shown to exist in TAA. The term "tubulopapillary hidradenoma" has been proposed for cases in which eccrine and apocrine differentiation are both observed. These tumors might develop from pluripotent appendageal cells7.
We present here an additional case of TAA associated with SCAP and this all developed in a NS. Further study is needed to understand the pathogenesis of these tumors.
Figures and Tables
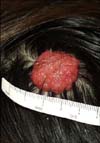 | Fig. 1A solitary, 2.5×2.5 cm well-circumscribed, erosive, erythematous lobulated nodule on the scalp. |
 | Fig. 2Syringocystadenoma papilliferum in the upper portion of the lesion and tubular apocrine adenoma in the lower portion (H&E, ×12.5). |
 | Fig. 3Findings of the upper part of the lesion. (A) Cystic and irregularly dilated tubular structures with deep invagination (H&E, ×200). (B) The lumina are lined by 2 layers of epithelial cells embedded in a fibroblastic stroma with a plentiful plasma cell infiltration (H&E, ×400). |
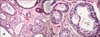 | Fig. 4Findings of the lower part of the lesion. (A) Variable sized tubular structures are seen (H&E, ×100). (B) Two layers of cells with evidence of decapitation secretion of the luminal cells are seen (H&E, ×400). |
References
5. Ansai S, Watanabe S, Aso K. A case of tubular apocrine adenoma with syringocystadenoma papilliferum. J Cutan Pathol. 1989. 16:230–236.

6. Ishiko A, Shimizu H, Inamoto N, Nakmura K. Is tubular apocrine adenoma a distinct clinical entity? Am J Dermatopathol. 1993. 15:482–487.

7. Aktepe F, Demir Y, Dilek FH. Tubular apocrine adenoma in association with syringocystadenoma papilliferum. Dermatol Online J. 2003. 9:7.

8. Ahn BK, Park YK, Kim YC. A case of tubular apocrine adenoma with syringocystadenoma papilliferum arising in nevus sebaceus. J Dermatol. 2004. 31:508–510.

9. Lee CK, Jang KT, Cho YS. Tubular apocrine adenoma with syringocystadenoma papilliferum arising from the external auditory canal. J Laryngol Otol. 2005. 119:1004–1006.

10. Yamane N, Kato N, Yanagi T, Osawa R. Naevus sebaceus on the female breast accompanied with a tubular apocrine adenoma and a syringocystadenoma papilliferum. Br J Dermatol. 2007. 156:1397–1399.

11. Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000. 42:263–268.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download