Abstract
Hydroa vacciniforme (HV) is a rare and chronic pediatric disorder that is characterized by photosensitivity and recurrent vesicles that heal with vacciniforme scarring. The pathogenesis of HV is unknown; no chromosome abnormality has been identified. HV patients have no abnormal laboratory results, so the diagnosis of HV is based on identifying the associated histological findings in a biopsy specimen and using repetitive ultraviolet phototesting to reproduce the characteristic vesicles on a patient's skin. Herein, we present a case of HV in a 7-year-old female who was diagnosed with HV according to histopathology and ultraviolet phototesting.
Hydroa vacciniforme (HV) is a very rare photodermatosis of unknown etiology that is principally seen in childhood. It is characterized by recurrent erythema and crops of papulovesicles or vesicles that appear on uncovered areas of skin within 1 to 2 days after sun exposure. These areas heal with deep hypopigmented scarring. By the late teenage years, this condition resolves spontaneously. The histopathologic findings of HV vesicles are distinctive and characterized by intraepidermal reticular degeneration and cellular necrosis. Reproduction of the vesicles with repetitive ultraviolet-A (UVA) phototesting may be an important diagnostic aid1.
We describe a case of HV that was diagnosed by reviewing the clinical features, laboratory evaluation, histopathologic findings and UVA phototesting of a 7-year-old female patient.
A 7-year-old Korean girl had suffered for 2 years from recurrent vesicles on her face, the dorsa of her hands and extensor surfaces of her forearms, with an associated feeling of warmth on those areas during both the spring and summer time. These skin lesions developed several minutes to hours after sun exposure. Each vesicle ruptured within 1 or 2 days, became crusted, and then gradually healed, leaving scars. There was no known exposure to photosensitizers, and the family history was negative for photosensitivity-related diseases.
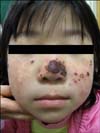
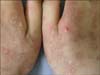
Physical examination showed erythematous, crusted and partially excoriated eczematous papules on the cheek and nose, which left hypopigmented and umbilicated scars (Fig. 1). Similar lesions were also present on the elbow and dorsa of her hands (Fig. 2).
The results of laboratory studies were all within normal limits, including the complete blood cell with differential count, platelet count, liver and renal function tests, red blood cell porphyrin levels, 24-hour fecal porphyrin levels and 24-hour uroporphyrin level. A skin biopsy of the cheek showed intraepidermal vesicles, spongiosis and epidermal necrosis with infiltration of dermal lymphocytes and neutrophils (Fig. 3). In addition, to rule out the presence of latent Epstein-Barr virus (EBV) infection or cutaneous EBV-associated lymphoproliferative disorders, immunostainings for EBV-determined nuclear antigens (EBNAs) and latent membrane proteins (LMPs) were conducted. No EBNAs or LMPs-positive cells were demonstrated in the lesional skin biopsy specimens (Fig. 4).
Phototesting with the minimal erythema dose (MED) of UVA and ultraviolet-B (UVB) was performed using a Waldmann UV3003K bank (Herbert Waldmann, Schwenningen, Germany). A UVB MED of 50 mJ/cm2 and a UVA of 40 J/cm2 were administered on the patient's back. Vesicles were induced at the MED test site exposed to 40 J/cm2 UVA (Fig. 5). We performed a skin biopsy on these UVA induced vesicles, and the histopathology showed only focal spongiosis and a superficial perivascular lymphocytic infiltration. The second skin biopsy showed mild histopathologic findings, so we asked the parents to allow their child to undergo the phototest again with a repetitive method to develop the typical vesicles; however, the parents refused it. Nevertheless, the results from the clinical features, histopathologic findings, laboratory data and phototesting heavily favored the diagnosis of HV. The patient was prescribed a topical sunscreen and told to visit the hospital if her skin lesions progressed. She was also advised to avoid direct exposure to sunlight.
HV, initially described by Bazin in 1862, was at first infrequently diagnosed because of the terminological confusion and uncertainty concerning the role of porphyrin metabolism in its pathogenesis2,3. At that point in time, some of the cases classified as HV had been protoporphyria until erythropoietic protoporphyria (EPP) was defined clearly4. HV is a very rare photodermatosis of unknown etiology that principally starts in childhood4. It has several distinctive features, including the (1) uniform development of vesicles and crusts several hours to 1 or 2 days after sun exposure, (2) healing of lesions with varioliform scarring, (3) absence of laboratory abnormalities, including serologic and porphyrin studies, (4) characteristic histopathology with epidermal necrosis and intraepidermal vesiculation, and (5) demonstrable evocation of the typical lesions by exposure to light5.
The differential diagnosis of HV consists of several blistering disorders that are light induced, including EPP, vesicular polymorphous light eruption (PMLE), bullous lupus erythematosus, solar urticaria, hydroa aestivale and porphyria cutanea tarda (PCT)4,5. Physicians can distinguish between these different illnesses in most cases by obtaining detailed historical, clinical, histopathologic and laboratory data. Although the vesicular form of PMLE is very rare, its clinical presentation may be similar to HV. However, in PMLE, the lesions almost always heal without scarring6, and the histological features differ from those of HV. The distinctive histologic changes of HV include initial intraepidermal vesicle formation with later focal epidermal keratinocyte necrosis and spongiosis in association with dermal perivascular neutrophil and lymphocyte infiltration. In vesicular PMLE, the dermal edema leads to subepidermal vesicle formation, mild to moderate epidermal spongiosis may occur, and epidermal necrosis is lacking7.
Hydroa aestivale is considered by some investigators to be a childhood type of PMLE, while other researchers have postulated that it is a nonscarring form of HV8,9, although hydroa aestivale has not been elicited by repetitive phototesting. The eruptions in EPP are typically an intensely edematous, urticarial reaction, and only its more severe purpuric and vesicular forms cause scarring. The histological features of EPP are deposition of a hyaline substance around the upper papillary blood vessels after repeated injury10. The urine, blood and stool porphyria laboratory results can help to exclude EPP and PCT. Bullous lupus erythematosus can be differentiated by a positive serologic profile and characteristic histology. The ability to reproduce the typical lesions in HV patients by phototesting has made this procedure a valuable diagnostic aid. In 1960, Schiff and Jillson were the first to document induction of lesions by phototesting that were clinically identical to those of HV, and phototesting with repetitive irradiation using a large dose of UVA has recently been shown to be very important for confirming the diagnosis of HV. Interestingly, our case showed that skin lesions were induced even with the MED of UVA, not with repetitive irradiation using a large dose of UVA.
Some reports have recently demonstrated that HV is associated with EBV infection and lymphoma, but some of these cases showed atypical features, and these cases may not represent the usual form of HV11.
In summary, we report here on a 7-year-old girl who had the typical skin lesions and histopathologic findings of HV. We induced vesiculation with the MED of UVA on her back, and the lesions showed mild histologic findings, yet we did not find any evidence of EBV infection in the skin biopsy sample.
Figures and Tables
Fig. 3
Histopathology from the erythematous vesicles on the patient's face showed epidermal necrosis, intraepidermal vesicles with spongiosis and a superficial perivascular lymphocytic infiltration (H&E stain, ×100).

Fig. 4
(A) There were no infiltrating mononuclear cells that expressed Epstein-Barr virus-determined nuclear antigens (EBNA) in the lesional skin biopsy specimen (×100). (B) There were no infiltrating mononuclear cells that expressed Epstein-Barr virus-latent membrane proteins (LMP) in the lesional skin biopsy specimen (×100).

References
1. Hann SK, Im S, Park YK, Lee S. Hydroa vacciniforme with unusually severe scar formation: diagnosis by repetitive UVA phototesting. J Am Acad Dermatol. 1991. 25:401–403.

2. Choi JH, Hann SK, Yoon MS, Choi BM, Ahn SK, Park YK. Hydroa vacciniforme: recurrence at adulthood and confirmative diagnosis by repetitive ultraviolet-A phototesting. Ann Dermatol. 1989. 1:83–86.
3. Sonnex TS, Hawk JL. Hydroa vacciniforme: a review of ten cases. Br J Dermatol. 1988. 118:101–108.

4. Hawk JLM, Ferguson J, Hönigsmann H. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Hydroa vacciniforme. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;820.
5. Eramo LR, Garden JM, Esterly NB. Hydroa vacciniforme. Diagnosis by repetitive ultraviolet-A phototesting. Arch Dermatol. 1986. 122:1310–1313.

7. Elpern DJ, Morison WL, Hood AF. Papulovesicular light eruption. A defined subset of polymorphous light eruption. Arch Dermatol. 1985. 121:1286–1288.

8. Redeker AG, Bronow RS. Erythropoietic protoporphyria presenting as hydroa aestivale. Arch Dermatol. 1964. 89:104–109.

9. Wheeler CE, Cawley EP, Whitmore CW. Hydroa aestivale in identical twins. Arch Dermatol. 1960. 82:590–594.

10. Goldgeier MH, Nordlund JJ, Lucky AW, Sibrack LA, McCarthy MJ, McGuire J. Hydroa vacciniforme: diagnosis and therapy. Arch Dermatol. 1982. 118:588–591.

11. Cho KH, Li KS, Kim YK, Jeon YK, Kim CW, Lee SK, et al. Epstein-Barr virus associated lymphoproliferative lesion presenting as a hydroa vacciniforme-like eruption. Korean J Dermatol. 2004. 42:846–855.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download