Abstract
Discoid lupus erythematosus (DLE), which is a cutaneous form of lupus erythematosus (LE), is generally refractory to a wide range of topical or systemic therapies. Although the main treatment option for DLE is topical steroids, it is often ineffective or likely to produce long-term side effects. New drugs, including tacrolimus and pimecrolimus, have been developed to overcome the adverse effects of steroids and treat the lesions of DLE for a prolonged period. We herein report 4 cases of facial DLE successfully treated with therapeutic adjuvants, topical tacrolimus or pimecrolimus.
Discoid lupus erythematosus (DLE) is one of the three types of lupus erythematosus (LE)-specific skin diseases, localized on the face and scalp in 80% of patients1. In the absence of systemic disease, the goals of therapy in a patient with DLE are improvement of the patient's appearance and prevention of deforming scars, atrophy or dyspigmentation2. Potent topical steroids are frequently prescribed, although their long-term use is often limited by adverse effects such as skin atrophy and telangiectasia3.
The compound macrolides, tacrolimus (FK506) and pimecrolimus (SDZ ASM 981), are members of a triad of calcineurin inhibitors that include cyclosporine A. They inhibit T-cell activation and the release of numerous inflammatory cytokines4. They are anti-inflammatory medications that might replace steroids for the topical treatment of many inflammatory skin diseases, including atopic dermatitis, contact dermatitis, pyoderma gangrenosum, psoriasis, alopecia areata, Behcet's disease, vitiligo, and lichen sclerosus, without having atrophogenic potential or risking other steroid-specific side effects4-10.
We herein report 4 cases of facial DLE successfully treated with therapeutic adjuvants, topical tacrolimus or pimecrolimus.
A 40-year-old woman presented with well-defined, scaly, erythematous plaques, which tended to heal with atrophy and scarring on her face. A skin biopsy from her cheek revealed hyperkeratosis, focal epidermal atrophy, basal cell degeneration and dilated follicular orifices filled with compact keratin. Patchy infiltrations of lymphocytes were observed in the dermis. Antinuclear antibody (ANA) was positive (1:160) with a speckled pattern. These findings were compatible with the characteristic features of DLE. She had received topical steroids for several years but showed no improvement.
She was treated with tacrolimus 0.1% ointment (Protopic®, Astellas Pharma, Tokyo, Japan) twice daily on the affected areas for 8 weeks. The amount of tacrolimus used was restricted to a thin layer applied to the affected areas (1.5 g/10 cm2). Additional topical therapy was restricted to the use of emollients and sunscreens. In order to evaluate the relapse rate, no other topical treatment was used for at least 8 weeks before initiation and after cessation of therapy.
Skin involvement before and after therapy was documented by photographs and semiquantitatively assessed by a clinical severity score. At each visit, the target lesions were assessed using 3 criteria: erythema, scale/thickness and scarring/atrophy. These 3 criteria were graded using a 4-point scale with a total score of 9 points (Table 1). Adverse events were recorded and included those reported by patients or observed by the medical staff. The total clinical severity score (0~9 points) was determined by adding together the 3 individual criteria scores. The overall assessment was expressed as excellent (improvement of total clinical severity score more than 75%), good (51~75%), fair (26~50%), and poor (less than 26%).
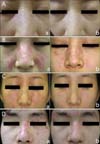
Topical tacrolimus was tolerated well without any side effects. There was a 75% reduction in the total clinical severity score, which was consistent with good improvement. Representative photographs before and after treatment are shown in Fig. 1A. Clinical improvement was sustained during the 4-week follow-up period after 8 weeks of treatment. Since completion of the study, the patient has been intermittently applying topical tacrolimus or pimecrolimus in combination with topical steroids; this treatment provides adequate control of her lesions.
An 18-year-old woman presented with disk-shaped erythematous plaques and depigmented scars on her face. A skin biopsy from her chin showed hyperkeratosis, basal cell degeneration and follicular plugging. These findings were consistent with DLE. She had failed to respond to a combination of potent topical steroids and oral antimalarial drugs.
She was treated with tacrolimus 0.1% ointment (Protopic®, Astellas Pharma, Tokyo, Japan) twice daily on the affected areas for 8 weeks. All other settings for clinical evaluation and scoring were identical to case 1. Topical tacrolimus was tolerated well without any side effects. There was a 57% reduction in the total clinical severity score, which was consistent with good improvement (Fig. 1B). Since completion of the study, she has been intermittently applying topical tacrolimus or pimecrolimus in combination with topical steroids; this treatment provides adequate control of her lesions.
A 25-year-old woman presented with indurated, scaly erythematous plaques on her face. She had not received any treatment for the plaques. A skin biopsy from the lesions revealed hydropic degeneration of the basal layer and an infiltration of inflammatory cells around the vessels in the dermis. These clinical and pathological findings were compatible with DLE. We applied pimecrolimus 1% cream (Elidel®, Novartis Pharma, Basel, Switzerland) twice daily on the affected areas for 8 weeks. All other settings for clinical evaluation and scoring were identical to case 1. There was a 63% reduction in the total clinical severity score, which was consistent with good improvement (Fig. 1C). Since completion of the study, she has been intermittently applying topical tacrolimus or pimecrolimus in combination with topical steroids; this treatment has provided adequate control of her lesions.
A 30-year-old man presented with well-demarcated, scaly, erythematous plaques on his face. A skin biopsy from the lesions revealed hyperkeratosis, focal epidermal atrophy and an infiltration of inflammatory cells around the adnexa in the dermis. These clinical and pathological findings were compatible with DLE. We applied pimecrolimus 1% cream (Elidel®, Novartis Pharma, Basel, Switzerland) twice daily on the affected areas for 8 weeks. All other settings for clinical evaluation and scoring were identical to case 1. He experienced burning sensation on the topically applied lesions at the beginning of treatment, but these symptoms resolved within a few hours without any further action such as cessation of treatment or application of other topical agents. There was a 71% reduction in the total clinical severity score, which was consistent with good improvement (Fig. 1D). Since completion of the study, he has been intermittently applying topical tacrolimus or pimecrolimus in combination with topical steroids; this treatment has provided adequate control of his lesions.
There are several case reports suggesting that tacrolimus and pimecrolimus provide good therapeutic efficacy for the cutaneous lesions of LE. Kanekura et al.11 assessed the efficacy of 0.1% tacrolimus ointment for a malar rash in 3 patients with systemic LE. After 3 weeks of twice-daily tacrolimus usage, all the lesions were gone. Druke et al.12 reported a case of subacute cutaneous LE, in which erythematous lesions were treated successfully with twice daily 0.1% topical tacrolimus application during an 8-week period. For patients with DLE, however, results thus far on the therapeutic efficacy of topical tacrolimus and pimecrolimus seem to be controversial. Yoshimasu et al.13 reported on the efficacy of 0.1% topical tacrolimus in 9 patients with LE. A good response was obtained for systemic LE, whereas the lesions of DLE did not improve. The lack of response in DLE cases was explained by the low absorption due to the hyperkeratotic and acanthotic changes present in chronic, scaly DLE lesions. However, recent studies have reported that DLE can be controlled by application of tacrolimus or pimecrolimus alone. For example, Walker et al.14 reported that a formulation of 0.3% tacrolimus in 0.05% clobetasol propionate ointment was effective in 2 patients with severe recalcitrant chronic DLE. Moreover, de la Rosa Carrillo and Christensen15 noticed both the improvement of facial lesions as well as the regrowth of terminal hair in most of the scalp lesions of DLE with tacrolimus monotherapy. Since then, a few studies have used severity scoring systems for clinical assessment. In Hefferman's open-label pilot study, 3 subjects applied 0.1% tacrolimus to 2 target lesions twice a day for 12 weeks16. The target lesions were assessed using 4 criteria: diameter size, erythema, scarring, and thickness. These 4 criteria were graded using a 5-point scale. The mean percentage improvement in scores for the 4 criteria was 32%, 53%, 56%, and 33%, respectively. In our case, the mean percentage improvements in scores for 3 criteria, which were erythema, scale/thickness, and scarring/atrophy, were 82%, 67%, and 33%, respectively. Similarly, Kreuter et al.17 performed a twice daily regimen for 3 weeks with 1% pimecrolimus cream in 11 subjects, including 4 DLE patients, and assessed the skin involvement with a clinical score using a 3-grade and 4-point scale before and after treatment. For all patients of the study, the overall percentage improvement in scores was 58%, but for the 4 patients with DLE it was 48%. Recently, Tlacuilo-Parra et al.18 used a severity score as well as the Skindex-29 for evaluation of the quality of life; their results showed a mean improvement of 46% in the quality of life of DLE patients after the use of tacrolimus.
Based on the good results reported in previous reports, we provided a twice-daily regimen of topical tacrolimus or pimecrolimus for 8 weeks and semiquantitatively assessed the skin involvement before and after therapy using a clinical severity score. After 8 weeks of therapy, both tacrolimus and pimecrolimus showed good responses in all patients (mean percentage improvement of all patients was 66.5%). The effects of treatment were more pronounced in our study, but this appears to be due to the fact that the majority of patients had lesions that showed erythema and scaling, as opposed to scarring and atrophy, which are seen in lesions that are more resistant to therapy. With regard to the efficacy of topical tacrolimus vs. pimecrolimus, no significant difference was found in the efficacy or side effects. Moreover, the improvement in skin condition was sustained in all patients for 4 weeks during the follow-up period. Since completion of the study, these 4 patients have been intermittently applying topical tacrolimus or pimecrolimus in combination with topical steroids; this treatment has provided adequate control of their lesions. However, the role of tacrolimus and pimecrolimus in the treatment of cutaneous LE will depend on its efficacy when compared to established topical treatments such as steroids. Therefore, double blind, placebo-controlled studies are needed in order to confirm our findings.
In conclusion, our reports suggest that tacrolimus 0.1% ointment and pimecrolimus 1% cream for patients with DLE seem to be safe and clinically effective options, especially for resistant DLE on a sensitive site, such as the face, where use of potent topical steroids carries a high risk for thinning of the skin and telangiectasia.
Figures and Tables
Fig. 1
Papulosquamous lesions of patients (cases 1: A, 2: B, 3: C, 4: D) with discoid lupus erythematosus before (a) and after treatment (b).
References
1. Rowell NR, Goodfield MJD. Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Systemic lupus erythematosus. Textbook of dermatology. 1998. 6th ed. Oxford: Blackwell Science;2461–2500.
2. Bongu A, Chang E, Ramsey-Goldman R. Can morbidity and mortality of SLE be improved? Best Pract Res Clin Rheumatol. 2002. 16:313–332.

3. Fabbri P, Cardinali C, Giomi B, Caproni M. Cutaneous lupus erythematosus: diagnosis and management. Am J Clin Dermatol. 2003. 4:449–465.
4. Grassberger M, Baumruker T, Enz A, Hiestand P, Hultsch T, Kalthoff F, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999. 141:264–273.

5. Eichenfield LF, Lucky AW, Boguniewicz M, Langley RG, Cherill R, Marshall K, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol. 2002. 46:495–504.

6. Ruzicka T, Bieber T, Schopf E, Rubins A, Dobozy A, Bos JD, et al. European Tacrolimus Multicenter Atopic Dermatitis Study Group. A short-term trial of tacrolimus ointment for atopic dermatitis. N Engl J Med. 1997. 337:816–821.

7. Harper J, Green A, Scott G, Gruendl E, Dorobek B, Cardno M, et al. First experience of topical SDZ ASM 981 in children with atopic dermatitis. Br J Dermatol. 2001. 144:781–787.

8. Ruzicka T, Assmann T, Homey B. Tacrolimus: the drug for the turn of the millennium? Arch Dermatol. 1999. 135:574–580.
9. Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol. 2002. 47:789–791.

10. Bohm M, Frieling U, Luger TA, Bonsmann G. Successful treatment of anogenital lichen sclerosus with topical tacrolimus. Arch Dermatol. 2003. 139:922–924.

11. Kanekura T, Yoshii N, Terasaki K, Miyoshi H, Kanzaki T. Efficacy of topical tacrolimus for treating the malar rash of systemic lupus erythematosus. Br J Dermatol. 2003. 148:353–356.

12. Druke A, Gambichler T, Altmeyer P, Freitag M, Kreuter A. 0.1% Tacrolimus ointment in a patient with subacute cutaneous lupus erythematosus. J Dermatol Treat. 2004. 15:63–64.

13. Yoshimasu T, Ohtani T, Sakamoto T, Oshima A, Furukawa F. Topical FK506 (tacrolimus) therapy for facial erythematous lesions of cutaneous lupus erythematosus and dermatomyositis. Eur J Dermatol. 2002. 12:50–52.
14. Walker SL, Kirby B, Chalmers RJ. The effect of topical tacrolimus on severe recalcitrant chronic discoid lupus erythematosus. Br J Dermatol. 2002. 147:405–406.

15. de la Rosa Carrillo D, Christensen OB. Treatment of chronic discoid lupus erythematosus with topical tacrolimus. Acta Derm Venereol. 2004. 84:233–234.

16. Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol. 2005. 141:1170–1171.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download