Abstract
Background
'Retinoid dermatitis' is a retinoid-induced irritant contact dermatitis (ICD). The mechanism of retinoid dermatitis may be different from that of other ICDs. However, it remains uncertain how topical retinoid induce ICD.
Objective
We compared several aspects of contact dermatitis induced by topical retinol and benzalkonium chloride (BKC) on hairless mice skin.
Methods
2% retinol or 2.5% BKC was applied to hairless mice and transepidermal water loss (TEWL), ear thickness, histologic and immunohistochemical findings were compared. We also compared mRNA expression of inflammatory cytokines, epidermal differential markers, cyclooxygenases (COXs) and heparin binding epidermal growth factor like growth factor (HB-EGF).
Results
Topical application of 2% retinol and 2.5% BKC increased TEWL and ear thickness in similar intensity. Epidermal hyperplasia was more prominent in retinol treated skin. Proliferating cell nuclear antigen, involucrin and loricrin expression were higher in retinol-treated skin than in BKC-treated skin. Filaggrin, however, was more expressed in BKC-treated skin. The mRNA expression of IL-8, TNF-α, COX-2, involucrin, loricrin and filaggrin were increased in both retinol- and BKC-treated skin in similar intensity. HB-EGF was more significantly increased in retinol-treated skin.
Many patients undergoing topical retinoid therapy experience retinoid dermatitis, a retinoid-induced irritant contact dermatitis (ICD) characterized by erythema, scaling, dryness, burning and pruritus, which in turn, result in discontinuation of topical retinoid treatment.
ICD is a cutaneous inflammation as a result of the direct cytotoxic effects of a chemical or physical agent. The pathophysiological pathway of ICD begins with penetration of the permeability barrier, damage of keratinocytes followed by the release of inflammatory mediators, such as IL-6, IL-8 and TNF-α1,2.
Retinoid dermatitis is considered to be induced by a mechanism distinct from that of other irritants and peeling agents3,4. Even though irritation induced by retinoic acid (RA) is generally considered a non-specific and undesirable adverse effect3, retinoic acid irritancy may be partly related to a receptor-mediated mechanism5,6.
RA bind to retinoic acid receptors (RARs) to activate the heterodimeric complex (RARs/RXRs) that binds to the retinoic acid response elements (RARE), thereby directly stimulating target gene transcription7. Among RAR proteins in the epidermis, nearly 90% are RAR-γ, with approximately 10% of RAR-α and no detectable RAR-β7. Chen et al.6 reported that RAR-γ mediated retinoid dermatitis in animal models. However, it remains unclear how retinoid and retinoid receptor interaction lead to retinoid dermatitis.
Recently, cytokines and cyclooxygenases (COXs) are thought to be involved in the induction of irritation. COX catalyzed the conversion of arachidonic acid (AA) to prostaglandin (PG) and exists in 2 isoforms, COX-1 and COX-28. COX-1 is expressed constitutively in most tissues and involved primarily in cellular homeostasis, whereas COX-2 is highly inducible and plays a major role in inflammation8,9.
It has been shown that heparin-binding epidermal growth factor-like growth factor (HB-EGF) can be induced by treatment with retinoids in human keratinocytes and organ-cultured skin, suggesting that epidermal hyperplasia following RA treatment may be mediated, at least in part, by keratinocyte-derived HB-EGF10. Other studies confirmed that HB-EGF mRNA was markedly induced by RA in normal human keratinocytes and normal mouse skin11-13. Chapellier's study also demonstrated a positive correlation between HB-EGF expression and RA-induced proliferation, further supporting the hypothesis that HB-EGF could be a paracrine factor synthesized in the suprabasal layers that mediates RA-induced hyperplasia12,13. In human skin, HB-EGF and amphiregulin have been reported to activate epidermal growth factor receptor (EGFR) and mediate retinoid-induced epidermal hyperplasia14.
The aim of this study was to elucidate the retinoid dermatitis specific features compared with ICD induced by benzalkonium chloride (BKC). In this study, we applied retinol and BKC on hairless mice and compared transepidermal water loss (TEWL), ear thickness, histopathologic findings, immunohistochemistry of epidermal proliferation and differentiation markers (proliferation cell nuclear antigen [PCNA], involucrin, loricrin, filaggrin). We also compared the mRNA expression of epidermal proliferation and differentiation markers, inflammatory cytokines (IL-8, TNF-α), enzymes associated with inflammation (COXs) and HB-EGF with reverse transcriptase-polymerase chain reaction (RT-PCR).
16 female hairless mice at the age of 6-weeks were used. Two groups of 8 mice were maintained at a temperature of 23±3℃, and a humidity of 50±10% in a controlled room. Throughout the study, the mice were fed on standard mouse pellets and water ad libtum.
In the preliminary studies, we found 2% all-trans-retinol (Sigma-Aldrich, St. Louis, MO, USA) and 2.5% BKC (Sigma-Aldrich, St. Louis, MO, USA) induced similar intensity of inflammation as measured by TEWL and ear thickness. Thus, 2% retinol and 2.5% BKC were used to compare irritant reaction induced by retinol and BKC. In the retinol-treated group, 2% retinol in ethanol was applied to the left ear and left back, and ethanol alone as a vehicle was applied to the right ear and right back. In the BKC-treated group, 2.5% BKC in acetone was applied to the left ear and left back, and acetone was applied to the right ear and right back. 20µl of each test compound was applied once daily for 4 consecutive days.
TEWL was measured from the back of hairless mice with Tewameter (Courage+Khazaka, Köln, Germany). The extent of edema induced by the skin irritants was measured by ear thickness with a dial thickness gauge (Peacock, Tokyo, Japan).
Paraffin sections were mounted on saline-coated microscopic slides (Muto Pure Chemicals, Tokyo, Japan) and washed serially in xylene and 100% ethanol for deparaffinization and rehydration. Endogenous peroxidase was inactivated with 3% H2O2 for 5 min followed by washing in ddH2O for 5 min. All of the following incubations were performed in a humidity chamber. The sections were incubated in 10 mM of sodium citrate buffer (pH 6.0) and microwaved for 10 min, then cooled in a refrigerator for 15 min and washed serially in ddH2O and PBS for 5 min. The primary antibodies used were monoclonal mouse anti-human PCNA (DAKO, Glostrup, Denmark), polyclonal rabbit anti-mouse involucrin (Convance Research Products, Denver, PA, USA), loricrin (Convance Research Products, Denver, PA, USA) and filaggrin (Convance Research Products, Denver, PA, USA). The sections were incubated with the primary antibodies diluted 1:100 for 1 hour at room temperature. Biotinylated secondary antibody with streptavidin-horseradish peroxidase was applied with a CAP-PLUS kit (Zymed, San Francisco, CA, USA) using the LAB-SA method. The sections were stained with the NovaRED substrate kit (Vector, Burlingame, CA, USA) and counterstained with hematoxylin.
Mouse ear and back skin tissue excised from each hairless mouse were incubated in 20 mM EDTA/PBS for 1 h at 37℃. The epidermis was separated from the dermis with fine forceps. RNA was extracted from the epidermis with Trizol reagent (Gibco BRL, Gaitherburg, MD, USA) containing acid-guanidinium thiocyanate-phenol chloroform. Extracted RNA was electrophoresed in formaldehyde denaturing gel to check 28s and 18s bands. Concentrations of samples were titrated arbitrarily to 1µg/ml using BioPhotometer (Eppendorf, Hamburg, Germany). RT-PCR was performed using RNA-PCR kit version 3.0 (Takara, Otsu, Japan). Briefly, cDNA amplification conditions are described as follows. Cycles of denaturing at 94℃ for 30 seconds, annealing at 60℃ for 30 seconds and extension at 72℃ for 90 seconds were repeated 35 times. PCR products were electrophoresed in 4% Nusieve 3:1 agarose gel (FMC, Rockland, ME, USA) and analyzed quantitatively using GelDoc2000 documentation system (Bio-Rad, Hercules, CA, USA). We utilized GAPDH and actin as house keeping genes. The primer sequences for cytokines, epidermal differentiation markers, COXs and HB-EGF were as follows: for IL-8 (KC), 5'-ATAACGCGTATGCAGCGCCTATCGCCAATGAGCTG-3' and 5'-CCAGATCTTACTTGGGGACACCTTT-3'; for TNF-α, 5'-GGCAGGTCTA CTTTAGAGTCATTGC-3' and 5'-ACATTCGAGGCTCCAGTGAATTCGG-3'; for COX-1, 5'-AGTCGCAGGAGTCTCTCGCTCTGG-3' and 5'-CAGGAAATGGGTGAACGAGGGGCT-3'; for COX-2, 5'-TTCAAAAGAAGTGCTGGAAAAGGTTCT-3' and 5'-AGATCATCTCTACCTGAGTGTCCTT-3'; for HB-EGF, 5'-TCCGTCTGTCTTCTTGTCATCGT-3' and 5'-TAGCCACGCCCAACTTCACT-3'; for filaggrin, 5'-CAGGGTCAGCGCAAGATC-3' and 5'-TGAGCCAGAGCTGGAACC-3'; for involucrin, 5'-CGGATATGGCAGGGGATC-3' and 5'-GGTGTAATTCTGGCTCACCAAG-3'; for loricrin, 5'-GGATCGTCCCAACAGTATCAG-3' and 5'-GACTGGTCT GCTGAGAGGAGTAAT-3'; for GAPDH, 5'-AATGGTGAAGGTCGGT GTGA-3' and 5'-CTGGAAGATGGTGATGGGC-3'; for actin, 5'-TGGAATCCTGTGGCATCCATGAAAC-3' and 5'-TAAAACGCAGCTCAGTAACAGTCCG-3'.
TEWL in both retinol- and BKC-applied skin is markedly increased but there is no significant difference between TEWL measured in retinol- and BKC-treated skin (Fig. 1).
Ear thickness is significantly increased in both retinol- and BKC-treated ears compared to ethanol- and acetone-treated ears. There was no significant difference between retinol- and BKC-treated mouse ear (Fig. 2).
H&E-stained sections from alcohol or acetone applied ears showed normal epidermal thickness with few dermal inflammatory cell infiltration, but sections from retinol- or BKC-applied ears showed epidermal hyperplasia, increased intercellular edema and dermal inflammatory cell infiltration. Epidermal hyperplasia and separation of the stratum corneum are markedly pronounced in retinol-applied skin, compared to BKC-applied skin (Fig. 3). Epidermal hyperplasia was also measured quantitatively by the average number of keratinocyte layers in the epidermis measured in three different locations in high power field (Fig. 4). The average number of keratinocyte layers is increased in both retinol- and BKC-applied ears compared to ethanol- and acetone-applied ears. The number of keratinocyte layers is significantly increased in retinol-applied skin compared to BKC-applied skin.
Immunohistochemical staining for PCNA to determine the extent of hyperproliferation showed that PCNA expressions in ethanol- or acetone-applied skin were normal. However, PCNA expressions in the basal layer of retinolor BKC-applied skin were increased. The increase was more pronounced in retinol-applied skin (Fig. 5).
We also performed immnunohistochemical staining for involucrin, loricrin, and filaggrin to determine the extent of epidermal differentiation. There was no remarkable increase in the expressions of these differentiation markers in ethanol- or acetone-applied skin, but the expressions of differentiation markers, such as involucrin, loricrin, and filaggrin were increased in the upper spinous layer and granular layer of retinol- or BKC-applied skin. Marked increase of involucrin and moderate increase of loricrin were observed in retinol-applied skin, compared to BKC-applied skin (Fig. 6). On the contrary, filaggrin was more pronounced in BKC-applied skin compared to retinol-applied skin.
Agarose gel electrophoresis of each cDNA associated with inflammation and keratinocyte growth (IL-8, TNF-α, COX-1, COX-2 and HB-EGF) amplified with RT-PCR is shown in Fig. 7A. No significant differences in COX-1 levels between the 4 groups were observed (Fig. 7A, B). IL-8, TNF-α, COX-2 and HB-EGF levels were significantly increased in retinol- or BKC-treated skin compared to vehicle-treated skin (Fig. 7A, C~F). Moreover, the HB-EGF level in retinol-treated skin was significantly increased compared to BKC-applied skin (Fig. 7A, F).
Agarose gel electrophoresis of each cDNA associated with keratinocytes differentiation (involucrin, loricrin and filaggrin) amplified with RT-PCR is shown in Fig. 8A. Involucrin, loricrin and filaggrin levels were significantly increased in retinol- or BKC-treated skin compared to vehicle-treated skin (Fig. 8B~D).
In this study, we found 2% retinol and 2.5% BKC induced similar level of barrier disruption and inflammation, as measured by TEWL and ear thickness, and we assumed that our model of ICD was adequate to compare different skin response to the application of retinol and BKC. As representative inflammatory mediators, we measured the profiles of inflammatory cytokines (IL-8, TNF-α) and COXs.
While resting keratinocytes produce some cytokines constitutively, a variety of environmental stimuli, such as tumor promoters, ultraviolet light and chemical agents can induce epidermal keratinocytes to release inflammatory cytokines (IL-1, TNF-α), chemotactic cytokines (IL-8, IP-10), growth promoting cytokines (IL-6, IL-7, IL-15, GM-CSF, TGF-α) and cytokines regulating immunity (IL-10, IL-12, IL-18). Many studies have been conducted to determine cytokine mRNA expression profiles in in vitro and in vivo ICD models2. Pro-inflammatory cytokine TNF-α15,16 and chemotactic cytokine IL-82,17 were up-regulated in ICD models in previous in vivo and in vitro studies, although most of the studies were performed in the early induction stage of irritant dermatitis. TNF-α and IL-8 were also reported to be up-regulated in retinoid dermatitis18-20. In our study, we observed that TNF-α and IL-8 up-regulation in both retinol- and BKC-induced dermatitis were consistent with that reported previously. There were no statistically significant differences in the levels of TNF-α and IL-8 up-regulation between retinol- and BKC-induced dermatitis.
The effect of retinoids on COXs is variable in different cell types. Xiao et al.21 reported that COX-2 protein expression was significantly increased by 9-cis retinoic acid, while COX-1 expression was not altered in scleroderma fibroblasts. On the other hand, in human skin squamous carcinoma cells which express COX-2 protein constitutively, 9-cis retinoic acid suppressed COX-2 expression and cell growth22. In our study, we observed no significant changes of COX-1 in retinol- or BKC-induced dermatitis, compared to ethanol- or acetone-treated controls, but we did observe a significant increase in COX-2 levels in both retinol- and BKC-induced dermatitis. There was no difference of COX-2 expression levels between retinol- and BKC-induced dermatitis.
Whereas the effects of retinoids on keratinocyte growth in cultures are variable, retinoids consistently stimulate keratinocyte growth in vivo7. Chapellier et al.12 demonstrated that topical retinoid signal was transduced by RXRα/RARγ heterodimers in suprabasal keratinocytes, which, in turn, stimulate the proliferation of basal keratinocytes via a paracrine signal that could be a heparin-binding EGF-like growth factor. Interestingly, HB-EGF does not possess any putative RARE. Therefore, direct modulation of HB-EGF by RARE promoters appears unlikely. Rather, RAR/RXR is likely to regulating other transcription factor that would, in turn, regulate HB-EGF gene expression23. Even though it is well known that irritants such as BKC induce epidermal hyperplasia, the underlying mechanism has not been thoroughly investigated as that in retinoids24,25. Scrape wounding, phorbol ester and various other stress stimuli on cultured cells are able to induce HB-EGF mRNA levels and the shedding of proHB-EGF into soluble HB-EGF26. In addition to EGF, IL-8 is also active as a mitogen for epithelial and endothelial cell proliferation26.
In our study, we observed a significant increase of HB-EGF expression in retinol-treated skin and in BKC-treated skin. Interestingly, expression of HB-EGF in retinol-treated skin was significantly increased than that in BKC-treated skin. This result is also consistent with markedly pronounced epidermal hyperplasia in retinol-induced ICD compared to BKC-induced ICD in our histopathological findings, and markedly increased expression of PCNA in our immunohistochemistry results. These results suggest that elevated HB-EGF and epidermal hyperplasia are more prominent, and the distinguished features of retinoid dermatitis although other ICD also showed elevated HB-EGF and epidermal hyperplasia. Abnormal proliferation of keratinocytes leading to pronounced epidermal hyperplasia and subsequent abnormal differentiation of the epidermis may lead to disruption of barrier function and inflammation in retinoid dermatitis.
Epithelial differentiation markers, including profilaggrin, loricrin, cornifin (small proline rich proteins), and transglutaminase 1 are also negatively regulated by retinoids in vitro27-30. On the contrary, retinoids up-regulate keratinocyte terminal differentiation markers in vivo31,32. Similarly, mild irritation by detergent, sodium dodecyl sulfate induced rapid and strong expression of the cornified envelope precursor protein involucrin in the stratum spinosum33. In our study, involucrin, loricrin and filaggrin levels were significantly increased in retinol- or BKC-applied skin compared to vehicle-applied skin as measured by RT-PCR and immunohistochemical stains.
The mechanism of retinoid dermatitis involves barrier disruption, cytokines, COX and HB-EGF induced epidermal hyperplasia. Although most of the factors mentioned above are also involved in ICD induced by BKC, relatively higher expression of HB-EGF and epidermal hyperplasia in retinoid dermatitis may be the characteristic features of retinoid dermatitis. Therefore, epidermal hyperplasia and elevated HB-EGF could be recognized as retinoid dermatitis specific feature. However, the underlying mechanism of retinoid dermatitis should be investigated in further.
Figures and Tables
 | Fig. 1Transepidermal water loss (TEWL) measured from the skin topically treated with retinol is significantly elevated than that measured in skin treated with ethanol. TEWL measured from the skin topically treated with benzalkonium chloride (BKC) was also significantly elevated, compared to that measured in skin treated with acetone. However, there was no significant difference between TEWL measured in retinol- and BKC-applied skin. Data are presented as mean±SEM, *p<0.01. Statistical significance was determined using Student's t test, empty box: ethanol-treated skin, shaded box: retinol-treated skin, striped box: acetone-treated skin, filled box: BKC-treated skin. |
 | Fig. 2Ear thickness is significantly increased in both retinoland benzalkonium chloride (BKC)-treated ears compared to ethanol- and acetone-treated ears, however, there is no significant difference between retinol- and BKC-treated ear. Data are presented as mean±SEM, *p<0.01. Statistical significance was determined using Student's t test, empty box: ethanol-treated skin, shaded box: retinol-treated skin, striped box: acetone-treated skin, filled box: BKC-treated skin. |
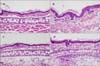 | Fig. 3Both retinol and benzalkonium chloride (BKC)-induced epidermal hyperplasia and inflammation. H&E-stained sections of ethanol-applied mouse ear (A), retinol-treated (B), acetone-treated ear (C), BKC-treated ear (D). Both retinol and BKC treatments result in epidermal hyperplasia, increased intercellular edema and dermal inflammatory cell infiltration (B and D). Epidermal hyperplasia and separation of the stratum corneum are more pronounced in retinol-applied ear skin compared to BKC-applied ear skin (H&E stain, ×400). |
 | Fig. 4Epidermal hyperplasia was also measured quantitatively by the number of keratinocyte layers in the epidermis. The number of keratinocyte layers is increased in both retinol- and benzalkonium chloride (BKC)-treated ears, compared to ethanoland acetone-treated ears. The number of keratinocyte layers is significantly increased in retinol-treated skin, compared to BKC-treated skin. Data are presented as mean±SEM, *p<0.01. Statistical significance was determined using Student's t test, empty box: ethanol-treated skin, shaded box: retinol-treated skin, striped box: acetone-treated skin, filled box: BKC-treated skin. |
 | Fig. 5Immunohistochemical stain - proliferation cell nuclear antigen (PCNA). (A) Normal epidermis, (B) Retinol, (C) Benzalkonium chloride (BKC). PCNA expressions in retinol- or BKC-applied skin are increased than those in normal or vehicle-applied skin. PCNA expression is more pronounced in retinol-applied skin than BKC-applied skin (×400). |
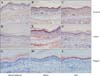 | Fig. 6Immunohistochemical stain - involucrin, loricrin, filaggrin. (A, D, G) Normal epidermis, (B) Involucrin in retinol, (C) Involucrin in benzalkonium chloride (BKC), (E) Loricrin in retinol, (F) Loricrin in BKC, (H) Filaggrin in retinol, (I) Filaggrin in BKC. Involucrin expressions in retinol- or BKC-treated skin are increased. Involucrin expression is slightly pronounced in retinol-treated skin, compared to BKC-treated skin. Loricrin expressions in retinol- or BKC-treated skin are increased. Loricrin expression is markedly pronounced in retinol-treated skin compared to BKC-treated skin Filaggrin expressions in retinol- or BKC-treated skin are increased. Filaggrin expression is slightly pronounced in BKC-treated skin compared to retinol-treated skin (×400). |
 | Fig. 7(A) Agarose gel electrophoresis of each cDNA amplified with reverse transcriptase-polymerase chain reaction (RT-PCR) (IL-8, TNF-α, COX-1, COX-2 and HB-EGF). (B) COX-1 levels by RT-PCR between 4 groups. No significant difference in COX-1 level between the 4 groups was observed (p=0.997, ANOVA). (C) IL-8 levels by RT-PCR between the 4 groups. IL-8 in retinol- or benzalkonium chloride (BKC)-applied skin is significantly increased compared to ethanol- or acetone-treated skin, respectively (p=0.012, p=0.006). However, no significant difference in IL-8 level between retinol- and BKC-treated groups was observed (p=0.157). (D) TNF-α levels by RT-PCR between the 4 groups. TNF-α in retinol- or BKC-treated skin is significantly increased compared to ethanol- or acetone-treated skin, respectively (p=0.001, p=0.046). No significant difference in TNF-α level between retinol- and BKC-treated groups was observed (p=0.460). (E) COX-2 levels by RT-PCR between the 4 groups. Unlike COX-1 results, COX-2 in retinol- or BKC-applied skin is significantly increased compared to ethanol- or acetone-treated skin, respectively (p<0.0001, p=0.005). No significant difference in COX-2 level between retinol- and BKC-treated groups was observed (p=0.221). (F) HB-EGF levels by RT-PCR between the four groups. HB-EGF in retinol- or BKC-treated skin is significantly increased compared to ethanol- or acetone-treated skin, respectively (p=0.01, p=0.0017). Moreover, HB-EGF in retinol-applied skin was significantly increased compared to BKC-treated skin (p=0.029). Data are presented as mean±SEM, *p<0.05, †p<0.01. Statistical significance was determined using Student's t-test, empty box: ethanol-treated skin, shaded box: retinol-treated skin, striped box: acetone-treated skin, filled box: BKC-treated skin. |
 | Fig. 8(A) Agarose gel electrophoresis of each cDNA amplified with reverse transcriptase-polymerase chain reaction (RT-PCR) (involucrin, loricrin, and filaggrin). (B) Involucrin in retinol- or benzalkonium chloride (BKC)-treated skin is significantly increased compared to ethanol- or acetone-treated skin respectively (p=0.017, p=0.020). However, no significant difference in involucrin level between retinol- and BKC-treated groups was observed. (C) Loricrin in retinol- or BKC-treated skin is significantly increased compared to ethanolor acetone-treated skin, respectively (p=0.025, p=0.0007). However, no significant difference in loricrin level between retinol- and BKC-treated groups was observed. (D) Filaggrin in retinol- or BKC-treated skin is significantly increased compared to ethanol- or acetone-treated skin, respectively (p=0.001, p=0.001). However, no significant difference in filaggrin level between retinol- and BKC-treated groups was observed. Data are presented as mean±SEM, *p<0.05, †p<0.01. Statistical significance was determined using Student's t test, empty box: ethanol-applied skin, shaded box: retinol-applied skin, striped box: acetone-applied skin, filled box: BKC-applied skin. |
References
1. Berardesca E, Distante F. Mechanisms of skin irritations. Curr Probl Dermatol. 1995. 23:1–8.
2. Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol Lett. 1998. 102-103:277–282.

3. Ale SI, Laugier JP, Maibach HI. Differential irritant skin responses to tandem application of topical retinoic acid and sodium lauryl sulphate: II. Effect of time between first and second exposure. Br J Dermatol. 1997. 137:226–233.

4. Kligman LH, Sapadin AN, Schwartz E. Peeling agents and irritants, unlike tretinoin, do not stimulate collagen synthesis in the photoaged hairless mouse. Arch Dermatol Res. 1996. 288:615–620.

5. Marks R, Hill S, Barton SP. The effects of an abrasive agent on normal skin and on photoaged skin in comparison with topical tretinoin. Br J Dermatol. 1990. 123:457–466.

6. Chen S, Ostrowski J, Whiting G, Roalsvig T, Hammer L, Currier SJ, et al. Retinoic acid receptor gamma mediates topical retinoid efficacy and irritation in animal models. J Invest Dermatol. 1995. 104:779–783.

7. Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996. 10:1002–1013.

8. Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996. 271:33157–33160.

10. Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp Dermatol. 1998. 7:391–397.

11. Yoshimura K, Uchida G, Okazaki M, Kitano Y, Harii K. Differential expression of heparin-binding EGF-like growth factor (HB-EGF) mRNA in normal human keratinocytes induced by a variety of natural and synthetic retinoids. Exp Dermatol. 2003. 12:Suppl 2. 28–34.

12. Chapellier B, Mark M, Messaddeq N, Calleja C, Warot X, Brocard J, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002. 21:3402–3413.

13. Xiao JH, Feng X, Di W, Peng ZH, Li LA, Chambon P, et al. Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J. 1999. 18:1539–1548.

14. Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006. 126:732–739.

15. Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992. 89:1398–1402.

16. Corsini E, Terzoli A, Bruccoleri A, Marinovich M, Galli CL. Induction of tumor necrosis factor-alpha in vivo by a skin irritant, tributyltin, through activation of transcription factors: its pharmacological modulation by anti-inflammatory drugs. J Invest Dermatol. 1997. 108:892–896.

17. Coquette A, Berna N, Vandenbosch A, Rosdy M, De Wever B, Poumay Y. Analysis of interleukin-1alpha (IL-1alpha) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol In Vitro. 2003. 17:311–321.

18. Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003. 146:65–73.

19. Dai X, Yamasaki K, Shirakata Y, Sayama K, Hashimoto K. All-trans-retinoic acid induces interleukin-8 via the nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways in normal human keratinocytes. J Invest Dermatol. 2004. 123:1078–1085.

20. Harant H, de Martin R, Andrew PJ, Foglar E, Dittrich C, Lindley IJ. Synergistic activation of interleukin-8 gene transcription by all-trans-retinoic acid and tumor necrosis factor-alpha involves the transcription factor NF-kappaB. J Biol Chem. 1996. 271:26954–26961.

21. Xiao R, Kanekura T, Yoshida N, Higashi Y, Yan KL, Fukushige T, et al. 9-Cis-retinoic acid exhibits antifibrotic activity via the induction of cyclooxygenase-2 expression and prostaglandin E2 production in scleroderma fibroblasts. Clin Exp Dermatol. 2008. 33:484–490.

22. Kanekura T, Higashi Y, Kanzaki T. Inhibitory effects of 9-cis-retinoic acid and pyrrolidinedithiocarbamate on cyclooxygenase (COX)-2 expression and cell growth in human skin squamous carcinoma cells. Cancer Lett. 2000. 161:177–183.

23. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw Hill;2108.
24. Fisher LB, Maibach HI. Effect of some irritants on human epidermal mitosis. Contact Dermatitis. 1975. 1:273–276.

25. Varani J, Perone P, Spahlinger DM, Singer LM, Diegel KL, Bobrowski WF, et al. Human skin in organ culture and human skin cells (keratinocytes and fibroblasts) in monolayer culture for assessment of chemically induced skin damage. Toxicol Pathol. 2007. 35:693–701.

26. Mathay C, Giltaire S, Minner F, Bera E, Herin M, Poumay Y. Heparin-binding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds. J Invest Dermatol. 2008. 128:717–727.

27. Floyd EE, Jetten AM. Regulation of type I (epidermal) transglutaminase mRNA levels during squamous differentiation: down regulation by retinoids. Mol Cell Biol. 1989. 9:4846–4851.

28. Asselineau D, Dale BA, Bernard BA. Filaggrin production by cultured human epidermal keratinocytes and its regulation by retinoic acid. Differentiation. 1990. 45:221–229.

29. Magnaldo T, Bernerd F, Asselineau D, Darmon M. Expression of loricrin is negatively controlled by retinoic acid in human epidermis reconstructed in vitro. Differentiation. 1992. 49:39–46.

30. Hohl D, de Viragh PA, Amiguet-Barras F, Gibbs S, Backendorf C, Huber M. The small proline-rich proteins constitute a multigene family of differentially regulated cornified cell envelope precursor proteins. J Invest Dermatol. 1995. 104:902–909.

31. Rosenthal DS, Griffiths CE, Yuspa SH, Roop DR, Voorhees JJ. Acute or chronic topical retinoic acid treatment of human skin in vivo alters the expression of epidermal transglutaminase, loricrin, involucrin, filaggrin, and keratins 6 and 13 but not keratins 1, 10, and 14. J Invest Dermatol. 1992. 98:343–350.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download