Abstract
Background
Pilomatricoma is a benign follicular tumor that is composed of basaloid cells, transitional cells and shadow cells. β-Catenin is a 92-kDa protein, and it plays important roles in cell-cell adhesion at the cell membrane and signal transduction in the nucleus. β-Catenin has recently been shown to play an important role in the formation of hair follicle-related tumors, including pilomatricoma. However, the pattern and the intracellular localization of the β-Catenin expression are still controversial.
Objective
We wanted to evaluate the pattern and the intracellular localization of the β-Catenin expression in pilomatricoma by performing immunohistochemical staining.
Methods
Twenty-seven paraffin-embedded tissue samples that were diagnosed as pilomatricoma were immunohistochemically stained with β-Catenin antibody.
Results
Basaloid cells were found 15 samples of the total 27 pilomatricomas. All (15/15) of the basaloid cells strongly expressed β-Catenin, but the transitional cells and the shadow cells did not. In the basaloid cells, the nuclei and membranes showed prominent β-Catenin immunoreactivities, but the cytoplasm showed weak β-Catenin immunoreactivity.
Pilomatricoma is a benign skin tumor that predominantly shows differentiation toward the matrical portion of a hair follicle at the level of the bulb1. Histopathologically, pilomatricoma is located in the dermis and subcutaneous tissue and this tumor is composed of basaloid cells, transitional cells, shadow cells and amorphous debris. Basaloid cells surround the periphery of the tumor, whereas transitional cells and shadow cells are scattered inside of the tumor in a form of islands. Although the process of differentiation of the tumor cells, including the basaloid cells, transitional cells and shadow cells, has been debated, it is know that the basaloid cells transform into shadow cells via the transitional cells with karyolytic changes2.
β-Catenin is a 92 kDa sized cytoplasmic protein, and it plays important roles in cell adhesion and signal transduction. At the cellular membrane, β-Catenin, as a subunit of the cadherin complex, links cadherin to the actin cytoskeleton to make up the cell-cell adherens junction3. The cadherin/β-Catenin complex is regulated by several mechanisms, including phosphorylation, and the cadherin/β-Catenin complex affects intercellular adhesion and affinity4. At the nucleus, β-Catenin controls the expression of genes in concert with Tcf/Lef transcription factors, and β-Catenin plays important roles in cell proliferation and differentiation5.
It has recently been reported that β-Catenin plays an important role in the pathogenesis of pilomatricoma6-8, but its exact role is still unknown. Several studies have tried to evaluate whether or not β-Catenin is involved in cellular proliferation and differentiation during the pathogenesis of pilomatricoma by examining the β-Catenin expression patterns in pilomatricoma9-13. However, each study showed different results for the β-Catenin expression patterns, that is, the intracellular patterns among the cell membrane, cytoplasm and nucleus, as well as the cellular patterns among the basaloid cells, transitional cells and shadow cells were slightly different according to the studies. Therefore, we evaluated the β-Catenin expression patterns in pilomatricoma by performing immunohistochemical studies.
Twenty seven paraffin-embedded tissue blocks that were obtained from biopsy specimens of histopathologically proven pilomatricoma were used in this study and 3 normal scalp specimens were used as controls. All the samples were from Yeungnam University Medical Center, Daegu, Korea, and the samples were obtained from January of 2007 to May of 2009.
Mouse anti-β-Catenin antibody (Clone CAT-5H10, C180226, Invitrogen Corporation, Camarillo, CA, USA) was used to study the β-Catenin expression patterns in pilomatricoma.
The paraffin-embedded tissue blocks were cut into 4µm sections and the tissue sections were stained with hematoxylin and eosin (H&E) to examine the histopathologic findings. The presence of tumor cells, including basaloid cells, transitional cells and shadow cells, was investigated along with the presence of inflammatory reactions. Foreign body reactions and calcifications in the stroma of the pilomatricoma were also evaluated.
To evaluate the β-Catenin expression patterns, the paraffin-embedded tissue blocks were cut in the same way as was done above. The tissue sections were stained using an automatic immunohistochemical stainer (BenchMark XT, Ventana Medical System, Inc., Tucson, AZ, USA). The programs of the automatic stainer included 1) deparaffinization, 2) rehydration, 3) heat-induced antigen retrieval (42℃, 30 minutes) and 4) a detection system with ultraView™ Universal DAB Detection Kit (Ventana Medical System, Inc., Tucson, AZ, USA). Then, the cellular and intracellular β-Catenin expression patterns of the tumor cells were evaluated.
On the H&E staining, 15 of the total 27 samples showed basaloid cells, transitional cells and shadow cells (Fig. 1), whereas 4 specimens showed transitional cells and shadow cells, and 8 specimens showed only shadow cells. Inflammatory reactions (17/27), foreign body reactions (17/27) and calcifications (14/27) were observed (Table 1). The inflammatory reactions were always accompanied by foreign body reactions.
On β-Catenin staining of the pilomatricomas (Fig. 2), all (15/15) of the basaloid cells exhibited prominent nuclear and membranous staining, but weak cytoplasmic staining (Fig. 3). The transitional cells (0/19) and shadow cells (0/27) did not express β-Catenin at all. Some of the inflammatory cells and endothelial cells showed irregular β-Catenin staining.
In the normal hair follicle of the healthy scalp skin, the β-Catenin immunostaining showed strong nuclear and membranous reactions, but weak cytoplasmic reactions of the hair matrix cells, whereas there were membranous reactions on the outer root sheath. Some of the hair papilla cells also exhibited nuclear immunostaining (Fig. 4). In the epidermis, the staining mainly showed membranous reactions along the intercellular junctions of the keratinocytes. Some of the sebocytes, endothelial cells and inflammatory cells also expressed β-Catenin.
Pilomatricoma is a benign follicular tumor and it histopathologically exhibits characteristic tumor cells such as basaloid, transitional and shadow cells. An electron microscopic study of pilomatricoma showed that the basaloid cells are morphologically the same as hair matrix cells14, and it implies that the differentiation of pilomatricoma proceeds in the same way as a normal hair follicle develops. During the development of pilomatricoma, basaloid cells are initially observed and transitional cells and/or shadow cells appear later, that is, the basaloid cells that fail to produce a normal hair follicle undergo karyolysis and they finally become shadow cells via transitional cells15,16. Some studies have suggested that bcl-2 and p53, which are apoptosis regulatory proteins, may play a role in the control of this abnormal apoptosis in pilomatricoma17,18. Lee et al.17 observed that bcl-2 and p53 were expressed only in the basaloid cells and apoptotic cells were found only in transitional cell zone, and they concluded that interaction between bcl-2 and p53 allows the basaloid cells to progress to shadow cells through the apoptotic transitional cells. In our study, basaloid cells were observed in 15 (55.6%) of the total 27 pilomatricomas on the H&E staining (Fig. 1). This result corresponds to that of Forbis and Helwig's study19, in which basaloid cells were observed in 124 (54.4%) of the total 228 pilomatricomas, yet the results of other studies have ranged between 72% and 100%20.
β-Catenin gene mutation has recently been shown to be an important regulator of the development and differentiation of hair follicles, and it is thought that the β-Catenin gene mutation plays an important role in the pathogenesis of pilomatricoma. Gat et al.6 confirmed that β-Catenin gene mutation was closely related to the development of follicular tumors in animal experiments using transgenic mice that expressed activated β-Catenin, and other studies revealed that β-Catenin gene mutations were observed in at least 75% of human pilomatricomas7,8.
Most studies have reported that the matrix cells in the normal hair follicles of the healthy scalp skin showed nuclear and cytoplasmic β-Catenin staining, but conflicting results have been reported for the β-Catenin staining of the outer and/or inner root sheath cells (Table 2)9-13. We think that these differences may the result of the differences of the employed anti-β-Catenin antibodies or stain techniques, yet we could not find any standardized method or guideline for β-Catenin staining in the literature. In our study, β-Catenin immunostaining of the normal hair follicles showed strong nuclear and membranous reactions, but weak cytoplasmic reactions of the hair matrix cells, whereas we observed membranous reactions of the outer root sheath. Some of the fibroblasts of hair papilla also exhibited nuclear immunostaining. Embryologically, complicated interactions between the epithelial cells and mesenchymal cells induce the development and differentiation of hair follicles21, but the delicate mechanisms are still unknown. Matrix cells of the hair bulb, via interaction with the adjacent hair papilla cells, differentiate into the medulla, cortex and cuticle of the hair and they finally produce hair shafts9. As the hair matrix cells and fibroblasts of hair papilla showed strong nuclear immunostaining in our study, it is supposed that the Wnt signaling pathway is involved in the development and differentiation of hair shafts. Nuclear translocation of β-Catenin is induced by the Wnt signal transduction pathway. In the absence of Wnt signaling, the level of β-Catenin is kept low through degradation of cytoplasmic β-Catenin that is in excess compared to the binding sites, such as the cadherins at the cell membrane22. β-Catenin mutation leads to increased levels of free β-Catenin in the cytoplasm and the nucleus and so this leads to blocked apoptosis, abnormal differentiation, cellular proliferation and finally oncogenesis23.
Park et al.10 observed that most transitional cells of pilomatricoma strongly expressed β-Catenin, but the basaloid cells and shadow cells did not, and that β-Catenin showed a prominent membranous immunoreactivity, but there was definitely no evidence of nuclear positivity, so they suggested that β-Catenin is primarily involved in cell-cell adhesion rather than cellular proliferation during the pathogenesis of pilomatricoma. However, most of the other studies observed that the basaloid cells of pilomatricoma showed prominent nuclear and membranous β-Catenin immunostaining, and the authors of those studies concluded that β-Catenin plays a key role in cellular proliferation and differentiation during the pathogenesis of pilomatricoma9,11-13. Xia et al.12 observed that there was no significance relationship between the rate of β-Catenin gene mutation and the strength of β-Catenin nuclear staining, but no β-Catenin mutations were detected in the pilomatricomas that were without nuclear β-Catenin staining. They suggested that the presence of β-Catenin nuclear localization is a useful indicator of the activation of the Wnt pathway in pilomatricomas. In our study, all (15/15) of the basaloid cells exhibited prominent nuclear and membranous β-Catenin staining, but weak cytoplasmic β-Catenin staining. The transitional cells (0/19) and shadow cells (0/27) did not express β-Catenin at all. These results imply that β-Catenin plays important roles not only in cell-cell adhesion, but also in cellular proliferation and differentiation during the pathogenesis of pilomatricoma via the Wnt signaling pathway. In addition, these patterns of the β-Catenin expression of the basaloid cells corresponded with those of the matrix cells of normal hair follicles.
The result of our study showing that the basaloid cells showed membranous β-Catenin immunostaining as well as nuclear and cytoplasmic β-Catenin immunostaining differed from the results of the other previous studies (Table 2)9-13. The results of all the studies, including ours, corresponded with each other in that the transitional cells did not express nuclear β-Catenin staining, but the results differed for whether the transitional cells expressed cytoplasmic and/or membranous β-Catenin staining.
In conclusion, we confirmed that the nucleus and membrane of all the basaloid cells showed a strong β-Catenin expression, and this result implies that β-Catenin plays important roles not only in cell-cell adhesion, but also in cellular proliferation and differentiation during the pathogenesis of pilomatricoma. Moreover, this pattern of the β-Catenin expression of pilomatricoma corresponded with that of the matrix cells, cortex cells and outer root sheath cells of normal hair follicles, and this suggests that pilomatricoma is a neoplasm that originates from the hair follicles that show follicular differentiation. A standardized method or guideline for β-Catenin staining has not yet been established, so further studies are needed to compare each β-Catenin staining method mentioned in Table 2 for obtaining more reliable results.
Figures and Tables
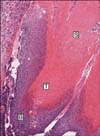 | Fig. 1Basaloid cells (B), transitional cells (T), and shadow cells (S) are observed in pilomatricoma (H&E, ×100). |
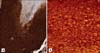 | Fig. 3Strong nuclear and membranous, weak cytoplasmic β-Catenin immunoreactivity in basaloid cells of pilomatricoma (A: ×200, B: ×400). |
 | Fig. 4(A) β-Catenin immunostaining of the normal hair follicles (×100). (B) Strong nuclear and membranous, but weak cytoplasmic β-Catenin immunoreactivity in matrix cells of normal hair follicle (×400). (C) Strong membranous and weak cytoplasmic β-Catenin immunoreactivity in outer root sheath cells of normal hair follicle (×400). (D) Strong nuclear β-Catenin immunoreactivity in fibroblasts of dermal papilla of normal hair follicle (×200). |
Table 2
Comparison of β-Catenin expression patterns in normal hair follicle and pilomatricoma and of immunohistochemical stain methods

*Park et al.10 (2001) and Demirkan et al.13 (2007) did not mention cellular localization of β-Catenin expression in normal hair follicle. †Nuclear and cytoplasmic β-Catenin expression was observed at central matrix cells and membranous β-Catenin expression at peripheral and basal matrix cells. ‡In IRS, membranous β-Catenin expression was observed at the level of hair bulb but absent at the level of hair shaft. N: nucleus, C: cytoplasm, M: membrane, ORS: outer root sheath, IRS: inner root sheath, -: negative.
References
1. Taylor RS, Perone JB, Kaddu S, Kerl H. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Appendage tumors and hamartomas of the skin. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1081–1082.
2. Choi YD, Park JN, Kang MS, Cho SH, Park SW. Expressions of cytokeratin and Ki-67 in the development of the pilomatricoma. Korean J Dermatol. 2003. 41:1619–1626.
3. Huber AH, Weis WI. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001. 105:391–402.

6. Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell. 1998. 95:605–614.

7. Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999. 21:410–413.

8. Durand M, Moles JP. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999. 86:725–726.
9. Moreno-Bueno G, Gamallo C, Perez-Gallego L, Contreras F, Palacios J. β-catenin expression in pilomatrixomas. Relationship with β-catenin gene mutations and comparison with β-catenin expression in normal hair follicles. Br J Dermatol. 2001. 145:576–581.

10. Park SW, Suh KS, Wang HY, Kim ST, Sung HS. β-Catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol. 2001. 145:624–629.

11. Hassanein AM, Glanz SM, Kessler HP, Eskin TA, Liu C. β-Catenin is expressed aberrantly in tumors expressing shadow cells. Pilomatricoma, craniopharyngioma, and calcifying odontogenic cyst. Am J Clin Pathol. 2003. 120:732–736.

12. Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue Y. beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006. 41:67–75.

13. Demirkan NC, Bir F, Erdem O, Düzcan E. Immunohistochemical expression of β-catenin, E-cadherin, cyclin D1 and c-myc in benign trichogenic tumors. J Cutan Pathol. 2007. 34:467–473.

14. Hashimoto K, Nelson RG, Lever WF. Calcifying epithelioma of Malherbe. Histochemical and electron microscopic studies. J Invest Dermatol. 1966. 46:391–408.
15. Seo SH, Jeong JT, Kye YC, Kim SN. The study of the clinical, histopathological and pathogenetic feature of pilomatricoma. Korean J Dermatol. 2001. 39:1275–1285.
16. Ackerman AB, Boer A, Bennin B, Gottlieb GJ. Histologic diagnosis of inflammatory skin disease: an algorithmic method based on pattern analysis. 2005. 3rd ed. New York: Ardor Scribendi;147.
17. Lee CW, Jang HS, Oh CK, Kwon KS. Apoptosis and expression of bcl-2, p53, and Ki-67 in pilomatricoma. Korean J Dermatol. 1999. 37:1560–1566.
18. Min KS, Shin JH, Lee HG, Kim JM. The immunohistochemical study of bcl-2 and p53 expression of pilomatricoma. Korean J Dermatol. 2000. 38:38–44.
19. Forbis R Jr, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961. 83:606–618.

20. Jang HZ, Baik YG, Kim JM, Sohn JH. The study of the clinical and histopatholgical features of pilomatricoma. Korean J Dermatol. 1997. 35:693–701.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download