INTRODUCTION
Chemical leukoderma is an acquired hypopigmented dermatosis induced by repeated exposure to chemicals. The most common chemicals are aromatic or aliphatic derivatives of phenols and catechols1,2. The clinical appearance and histopathologic finding may be indistinguishable from idiopathic vitiligo. There are no confirmatory tests for chemical leukoderma. Long-term follow-up studies indicate that in some cases of chemical leukoderma, even after avoiding the offending chemicals strictly for more than 1 year, vitiliginous patches may continue to develop in different parts of the body1. Treatment of chemical leukoderma is similar to that of idiopathic vitiligo. We report a case of chemical leukoderma that developed after repeated exposure to industrial chemicals.
CASE REPORT
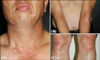
A healthy 51-year-old man presented with multiple hypopigmented macules and patches on his face, neck, arms, and legs (Fig. 1). He had no past medical history including autoimmune disease. His family history was unremarkable. He had worked in a factory for 1 year and 8 months producing materials for automobiles. He mixed adhesives, including resin powders, and chemicals together with raw materials such as fiber fragments. Despite wearing dustproof mask and wristlets, his face and neck areas had been exposed to a large amount of dusts. Four months after starting this job, he began to develop pruritic erythematous patches on his arms. His skin lesions were sometimes relieved by work interruption, especially during the winter season. He had been treated with topical steroids with some improvement. After 10 months, hypopigmented macules developed on the face, neck, arms and legs, including previous inflammatory sites, such as arms. His colleagues at work had similar skin lesions, but he exhibited the most severe reaction among other workers. He left his job 2 months before visiting our outpatient clinic, with enlargement of hypopigmented patch lesions. Topical corticosteroid was ineffective for his skin lesions.
There were no abnormalities besides the skin lesions on general physical examination at his visit. Routine laboratory studies performed in another hospital including complete blood count, liver function test, renal function test and urinalysis were all within the normal limits.
As result of material safety data sheet analysis, phenol, phenolic resin, formalin and hexamethylenetetramine were detected in his former work environment. We performed patch test with Korean standard series, cosmetic series, fragrance series, as well as phenol 1%, phenolic resin 1% and hexamethylenetetramine 1%. Patch test revealed positive result to fragrance mix, balsam of Peru, hexamethylenetetramine 1% (Fig. 2).
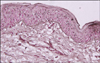
With a clinical diagnosis of chemical leukoderma, punch biopsy was performed on hypopigmented lesion. H&E staining showed interface dermatitis. Dopa staining showed loss of dopa positive cell (Fig. 3).
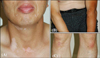
The patient was treated with mid-potency topical steroid ointment for 4 weeks, but the lesions remained unchanged. He declined phototherapy because of personal reason and steroid pulse therapy was therefore started. After being treated with prednisolone 20 mg for 1 week, it was discontinued for 3 weeks. By the same treatment schedule, 3 periods of steroid pulse therapy had been administered. Following treatment, the lesions were much improved in number and size (Fig. 4). He did not complain of any treatment-related side effects. He is taking topical corticosteroid treatment and currently being followed-up at our outpatient clinic. Although much improvement is observed at his last visit 5 months following treatment initiation, some skin lesions remained.
DISCUSSION
The first case of toxic leukoderma following occupational contact was reported in 1939, in workers exposed to monobenzyl ether of hydroquinone present in rubber gloves3. Since then, a variety of chemicals causing chemical leukoderma have been reported. The majority of these chemicals are aromatic or aliphatic derivatives of phenols and catechols1,4-6. Other contributory toxins are corticosteroids, tretinoin, sulfhydryls, mercurials, arsenics, cinnamic aldehyde, p-phenylenediamine, azelaic acid, systemic medications such as chloroquine1. These chemicals are injurious to melanocytes only in subjects having specific genetic susceptibility. In Korea, the most common occupational skin disease is contact dermatitis. Leukoderma constitutes about 5.7% of occupational skin diseases7.
Leukoderma can occur due to the toxic effects of a specific chemical or preceding allergic contact dermatitis. The mechanism is either destruction or inhibition of melanocytes by the offending substance. Leukoderma does not cause physical functional defects. It can, however, cause esthetic problems such as psychological distress and social problem. The areas of involvement depend on the route of exposure. Lesions are frequently widespread, including areas of direct skin contact and accidentally transferred from hand to other parts of body. Systemic absorption by accidental ingestion or percutaneous absorption has been hypothesized to explain some cases in which lesions occurred on unexposed skin1.
No diagnostic criteria can differentiate chemical leukoderma from vitiligo clinicohistopathologically. However, chemical leukoderma can be diagnosed clinically by a history of repeated exposure to a known or suspected depigmenting agent at the primary site, in addition to the presence of numerous acquired pea-sized macules, and distribution of macules corresponding to chemical exposure. Pruritus is sometimes present in chemical leukoderma, as in our case but usually not in vitiligo1.
Our patient had no history of vitiligo, autoimmune disease and specific family history. Contact dermatitis lesions were first developed in occupationally exposed sites after 10 months of commencing work. In the patch test, he showed positive result to fragrance mix, balsam of Peru, hexamethylenetetramine 1%, the latter of which he contacted in his work environment might be the causative agent of his previous allergic contact dermatitis, which was easily controlled with topical corticosteroids initially. After 10 months, hypopigmented macules developed on more extensive areas, including previous inflammatory sites but most hypopigmented areas developed on the previously normal areas. Dopa staining showed melanocyte depletion. Phenol, which the patient had contact with in his occupation is well known to cause leukoderma and vitiligo5,6,8-10. Based on these findings, chemical leukoderma was diagnosed and managed with topical corticosteroid. The lesions were refractory treatment. After 1 cycle of systemic steroid pulse therapy, the lesions stopped progressing and began to shrink. He completed 3 cycles of systemic steroid pulse therapy as scheduled and achieved repigmentation in more than 50% of lesions.
In localized chemical vitiligo, topical steroid is usually the first treatment option. However, in generalized vitiligo, psoralen and ultraviolet A, narrowband ultraviolet B phototherapy, 308 nm excimer laser therapy, systemic steroid, cyclosporine, other immunosuppressive agents and combination therapy of 2 or 3 treatment modalities may be used, according to disease activity and site11. There is no agreed treatment guideline for chemical leukoderma. Strict avoidance of the offending chemicals was most important. Oral dexamethasone pulse treatment12,13, low-dose oral corticosteroids (0.3 mg/kg body weight) for vitiligo showed good response14. In leukoderma, there are no published outcomes on systemic steroid therapy. Because the prognosis and treatment response of chemical leukoderma were better than that of vitiligo, we treated the patient with 3 cycles of low dose systemic steroid (0.3 mg/kg body weight) pulse therapy, which resulted in good response without any side effects. Further studies are required to determine the appropriate dose and treatment schedule for systemic steroid pulse therapy in chemical leukoderma.
In conclusion, systemic steroid pulse therapy may be a good treatment option for chemical leukoderma when avoidance of the causative chemicals and other topical treatments are ineffective.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download