Abstract
Anti-tumor necrosis factor (TNF)-α agents promise better disease control for the treatment of ankylosing spondylitis resistant to classical disease-modifying treatments. Etanercept, a recombinant human TNF receptor fusion protein, is used to treat a variety of TNF-α-mediated diseases by inhibiting the biological activity of TNF-α. We experienced a case of pustular psoriasis in a 32-year-old man during anti-TNF-α therapy with etanercept. He had a history of ankylosing spondylitis for 2 years. Two years after treatment of etanercept, erythematous pustules developed on his palms and soles. He had no previous history of pustular psoriasis. The skin lesion improved as the etanercept therapy was stopped, but pustular skin eruption recurred as adalimumab, a different TNF-α inhibitor, was administered to manage his ankylosing spondylitis. Several TNF-α inhibitors have different molecular structures, but these inhibitors might have a similar potency to induce pustular psoriasis from this case.
Psoriasis is a chronic inflammatory disease that occurs in about 0.1% to 3% of the population and is characterized by T cell-mediated cytokine production that drives hyperproliferation and abnormal differentiation of keratinocytes1. The incidence of pustular psoriasis is very low. Factors that effect occurrence and deterioration in psoriasis are skin trauma, mental and physical stress, cold, dry climate, excessive alcohol intake and drugs. Drugs are usually involved in the occurrence of a new lesion, in the absence of a family or past history of psoriasis. Based on Psoriatic Drug Eruption Probability Score, beta-blockers, synthetic anti-malaria drugs, non-steroidal anti-inflammatory drugs and tetracycline antibiotics are relevant with psoriasis2. Interestingly, TNF-α inhibitors, used in the treatment of severe psoriasis and psoriatic arthritis, contribute to the development of psoriasiform eruptions and psoriasis3.
We experienced a case of pustular psoriasis during anti-TNF-α therapy with etanercept for treatment of ankylosing spondylitis. The pustular skin eruption recurred when adalimumab, a different TNF-α inhibitor, was administered, instead of etanercept, to manage ankylosing spondylitis. Several TNF inhibitors have different molecular structures, but these inhibitors might have a similar potency to induce pustular psoriasis from this case.
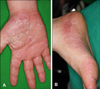
A 32-year-old man with rheumatoid arthritis and no personal or family history of psoriasis was treated with methotrexate and etanercept. Two years after the initiation of TNF-α inhibitor therapy, he developed an erythematous pustular eruption on his palms and soles (palmoplantar pustulosis) that evolved into psoriasiform changes (Fig. 1). The skin biopsy specimen showed psoriasiform epidermal hyperplasia with hyperkeratosis and confluent parakeratosis. There were a few telangiectatic blood vessels in the papillary dermis associated with a perivascular lymphocytic infiltration (Fig. 2). We considered the appearance of a skin lesion as an adverse event to etanercept. Consequently, etanercept treatment was discontinued, and the patient was treated with actretin in combination with a topical steroid. The skin lesion improved remarkably (Fig. 3). Due to a flare-up of joint symptoms, however, he restarted etanercept treatment, which induced pustular skin eruption again. Instead of etanercept, he was treated with adalimumab, a different TNF-α inhibitor, to manage his ankylosing spondylitis. But, a mild degree of pustular skin eruption developed again with the adalimumab therapy (Fig. 4).
TNF has many effects on the immune system (Table 1). TNF-α inhibitors are used to treat chronic autoimmune diseases and inflammatory conditions, including psoriasis. The complete mechanism of action remains unclear. These inhibitors are suppressed by pro-inflammatory cytokines such as interleukin-8 (IL-8), IL-6 and colony-stimulating factors and by reduced infiltration of neutrophils, T cells and plasmacytoid dendritic cells (PDCs) in the epidermis and papillary dermis4.
The most common side effects of TNF-α inhibitors are mild to moderate degrees of itching, pain, swelling and redness at the site of injection. Cutaneous adverse events of TNF-α inhibitors, such as eczematoid dermatitis, cutaneous lymphoma, herpes simplex infection, bacterial infection, lichenoid eruption, erythema multiforme, lupus erythematosus and acute generalized exanthematous pustulosis, have been reported5.
Paradoxically, TNF-α inhibitors may induce or aggravate psoriasisform eruption and palmoplantar pustular psoriasis1,3,6. The occurrence of pustular lesions ranges from a few days to years after administration, and gender and age are not related1. The incidence of TNF-α inhibitor-induced psoriasis was estimated at 2.3 to 5% in patients1. More than half of these patients presented with palmoplantar pustules1. The mechanisms underlying the paradoxical event remain elusive, but PDCs and INF-α seem to be key factors.
TNF-α has been shown to regulate INF-α production and also to inhibit the maturation of PDCs from hematopoietic progenitors. Inhibition of TNF-α may allow abnormal production of INF-α by PDCs1. Clinical evidence between psoriasis and INF-α has been reported6. After injection of recombinant IFN-α, psoriasis was aggravated; application of imiquimod cream worsened psoriasis. PDCs were found to infiltrate the skin of psoriasis7, and IFN-α increased in the lesional dermal vasculature and perivascular lymphocytic infiltration1. Accordingly, suppressing TNF-α induces PDCs-derived INF-α production, which leads to a paradoxical reaction.
There are several differences between etanercept and adalimumab (Table 2). TNF is a cytokine that includes soluble TNF (sTNF) and transmembrane TNF (tmTNF), consisting of type I (p55) and type II (p75) receptors8. Etanercept is a recombinant fusion protein that consists of the soluble TNF receptor (p75) linked to the Fc portion of human immunoglobulin G1 (sTNFR II/IgG1 Fc fusion protein, sTNFR:Fc). It is administered as a subcutaneous injection. Etanercept is a receptor blocker that binds to both sTNF and tmTNF, but only to the trimer form of sTNF9. Etanercept-sTNF complex showed to be relatively unstable. Etanercept dose did not induce complement-mediated cell lysis10. Adalimumab is a human immunoglobulin G1 monoclonal antibody that binds, with both trimer and monomer forms of soluble TNF, to form a stable complex with sTNF and tmTNF9. Adalimumab is administered as a subcutaneous injection. It may induce complement-mediated cell lysis due to its capability of complement fixation11,12. The capacity of cell-binding is 3 folds higher by adalimumab than by etanercept4. Adalimumab initiated apoptosis through tmTNF, but etanercept did not11.
The difference in structure and pharmacokinetic properties could explain the different response to TNF inhibitors. Switching among TNF-α inhibitors can promise significant clinical benefits including reduced side effects and increased effectiveness13-16. In contrast, this case serves as a good example of TNF-α inhibitors having a potential to induce adverse effects by cross-reaction.
Figures and Tables
Fig. 1
After 2 years of etanercept therapy for ankylosing spondylitis, erythematous scaly pustular lesions arose on both palms (A) and soles (B).
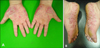
Fig. 2
Epidermal hyperplasia with hyperkeratosis and parakeratosis are shown on horny layer; granular layer has disappeared (A, B). Capillaries in the papillary dermis associated with perivascular lymphocytic infiltration (B). Munro microabscess was shown. Intraepidermal pustule formation was shown (C) (H&E, A: ×40, B: ×100, C: ×200).
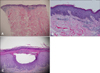
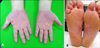
Fig. 3
Erythematous scaly patches on both palms (A) and soles (B) have improved following etanercept discontinuation.
References
1. de Gannes GC, Ghoreishi M, Pope J, Russell A, Bell D, Adams S, et al. Psoriasis and pustular dermatitis triggered by TNF-alpha in patients with rheumatologic conditions. Arch Dermatol. 2007. 143:223–231.
2. Dika E, Varotti C, Bardazzi F, Maibach HI. Drug-induced psoriasis: an evidence-based overview and the introduction of psoriatic drug eruption probability score. Cutan Ocul Toxicol. 2006. 25:1–11.

3. Choi YJ, Kim DS, Park JM, Oh SH, Park YK, Lee JH. A case of psoriasiform eruption triggered by tumor necrosis factor-alpha antagonist therapy. Korean J Dermatol. 2008. 46:721–723.
4. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008. 117:244–279.

5. Romero-Mate A, Garcia-Donoso C, Cordoba-Guijarro S. Efficacy and safety of etanercept in psoriasis/psoriatic arthritis: an updated review. Am J Clin Dermatol. 2007. 8:143–155.
6. Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008. 59:996–1001.

7. Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005. 202:135–143.

9. Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002. 359:1187–1193.

11. Solau-Gervais E, Laxenaire N, Cortet B, Dubucquoi S, Duquesnoy B, Flipo RM. Lack of efficacy of a third tumour necrosis factor alpha antagonist after failure of a soluble receptor and a monoclonal antibody. Rheumatology (Oxford). 2006. 45:1121–1124.

12. Haibel H, Brandt HC, Song IH, Brandt A, Listing J, Rudwaleit M, et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis. 2007. 66:419–421.

13. Lee JG, Park HJ, Lee YA, Hong SJ, Lee SH, Yang HI. Clinical improvement of ankylosing spondylitis on switching tumor necrosis factor inhibitors: a case report. Korean J Med. 2008. 75:S930–S934.
14. Laas K, Peltomaa R, Kautiainen H, Leirisalo-Repo M. Clinical impact of switching from infliximab to etanercept in patients with rheumatoid arthritis. Clin Rheumatol. 2008. 27:927–932.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download