Abstract
Background
Melasma is a common acquired symmetrical hypermelanosis that occurs on sun-exposed areas, and it is frequently observed among women. Various treatment modalities have been tried, but none are completely satisfactory. 4-n-butylresorcinol, which is a resorcinol derivative that has an inhibitory effect on both tyrosinase and tyrosinase-related protein-1, was introduced in 1995 and it has received increasing attention as a new hypopigmenting agent. However, the hypopigmenting effect of 4-n-butylresorcinol in human subjects has only been shown in a few studies.
Objective
The aim of this study was to investigate the hypopigmenting efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma.
Methods
Twenty patients with melasma were enrolled to this randomized, double-blind, vehicle-controlled, split-face comparative study. The patients were instructed to apply 4-n-butylresorcinol 0.1% cream or vehicle to each side of the face twice daily for 8 weeks. Mexameter measurements were performed along with photography at baseline, 4 weeks and 8 weeks. Adverse events were observed and recorded throughout the study.
Results
All the patients completed the study. Mexameter measurements demonstrated that the melanin index of the treated side showed a significant decrease when compared with that of the vehicle-treated side after 4 weeks (p=0.006) and after 8 weeks (p<0.0005). All the adverse reactions were mild and transient.
Melasma is an acquired hyperpigmentary disorder that shows a symmetric distribution on sun-exposed areas, and it is predominantly seen on the face. It is common in women of child-bearing age. Sun exposure, oral contraceptive pills, pregnancy, endocrine dysfunction and a genetic predisposition have all been implicated in the etiology of melasma. Among these, exposure to ultraviolet radiation is thought to be the most important factor.
It is well known that tyrosinase plays key role in melanogenesis as it regulates the rate-limiting step of melanogenesis1,2. Thus, tyrosinase inhibition is one of the major strategies when developing new hypopigmenting agents. The most commercially available hypopigmenting agents include tyrosinase inhibitors as the major active ingredients. Hydroquinone, kojic acid and arbutin are most commonly used and often used in combination with other therapies such as retinoic acid, topical corticosteroids or laser treatment to achieve a synergistic effect. However, none have shown satisfactory results.
In 1995, Okubo et al.3 reported that 4-n-butylresorcinol has an inhibitory effect on the melanogenesis of cultured B16 melanoma cells by its direct inhibition of tyrosinase activity, as well as the suppression of tyrosinase synthesis, without inducing any cytotoxicity. Since then, further in vitro studies have demonstrated that 4-n-butylresorcinol inhibits melanin production as well as the activity of both tyrosinase and tyrosinase-related protein-1 (TRP-1)4-7.
Despite the increasing attention paid to this agent, only a few clinical trials have demonstrated the hypopigmenting effect of 4-n-butylresorcinol5,8-10. In addition, skin irritation can be induced by 4-n-butylresorcinol, which is a resorcinol derivative, and particularly when it is applied in high concentrations11.
Among the various methods, a lower concentration of 4-n-butylresorcinol can be used to decrease the chance of skin irritation. The aim of this study was to investigate the hypopigmenting efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma.
We conducted a randomized, double-blind, vehicle-controlled, split-face comparison study of 4-n-butylresorcinol 0.1% cream for the treatment of melasma. This study was approved by our Institutional Review Board. All the patients provided informed written consent prior to their participation. The patients were healthy female subjects aged 20 years or older with a diagnosis of melasma. The Fitzpatrick skin type was recorded for all the patients. The exclusion criteria included pregnancy, breastfeeding, infectious skin disease, serious medical disorders and recent hormone or corticosteroid therapy. At the baseline visits, the patients were given two products that were similarly packaged: one contained vehicle plus 4-n-butylresorcinol 0.1% cream and the other contained vehicle alone. They were informed to use a standard amount of each cream with the fingertip-unit12 and apply the two formulations to each half of their face, twice daily, for 8 weeks. The side of face receiving each product was randomly assigned for each patient. The patients were not allowed to use any bleaching agents during the study. They were also instructed to avoid sun exposure and apply a broadspectrum sunscreen.
The patients visited our department 3 times (at baseline, week 4 and week 8), and objective skin color measurements were performed using a Mexameter® (MX-18; Courage & Khazaka Electronic GmbH, Cologne, Germany) during each visit. Three successive measurements of the melanin index (MI) were made on the same darkest portion of the 4-n-butylresorcinol-treated skin and the vehicle-treated skin. The mean value of the data obtained from each half of the face was then calculated and compared. Additionally, photographs of the patients were taken at baseline, week 4 and week 8.
The potential adverse effects were self-reported by the patients at any time. Clinically, the investigator graded the degree of erythema, scaling, itching and burning at each visit using a 0~3 scale (0, none; 1, mild; 2, moderate; 3, severe).
For statistical analysis, an independent samples t-test was used to compare the change in the mean MI resulting from treatment between the 4-n-butylresorcinol-treated and vehicle-treated hemi-faces. The data was analysed using SPSS software (SPSS V12.0K, SPSS Inc., Chicago, IL, USA). p-values<0.05 were considered statistically significant.
Twenty melasma patients were enrolled. All the patients were Korean women, and their mean age was 40.40±6.03 years (age range: 28~49 years). The Fitzpatrick skin type was III in 10 (50%) patients, V in 6 (30%) patients and IV in 4 (20%) patients. All the patients completed the trial.
After 4 weeks of application, a statistically significant decrease in the mean melanin index (MI) of the 4-n-butylresorcinol-treated skin (-3.43%) was observed compared with the vehicle-treated skin (-0.15%) and as measured by the Mexameter® (p=0.006). After 8 weeks, the mean MI of the 4-n-butylresorcinol-treated skin also showed a significant decrease (-4.87%), whereas the mean MI of the vehicle-treated skin showed a slight increase (+2.21%) (p<0.0005) (Table 1).
The changes in the mean melanin index during the 8-week study period are shown in Fig. 1. The representative photographs showing the improvement observed on three patients with different degrees of reduction of the melanin index treated with 4-n-butylresorcinol 0.1% cream are shown in Fig. 2, 3, 4.
The side effects were very limited. After 4 weeks, mild erythema and itching were seen in 2 (10%) patients, which was all self-limiting. After 8 weeks, no adverse events related to the study product were observed by the investigators or experienced by the patients.
Melasma is a chronic distressing condition that is often therapeutically challenging. 4-n-butylresorcinol is a newly developed hypopigmenting agent that has an inhibitory effect against both tyrosinase and TRP-1. The hypopigmenting efficacy of 4-n-butylresorcinol has been demonstrated through several in vitro and in vivo studies since it was first reported on in 19953-10. In an 18-week, placebo-controlled clinical study, 4-n-butylresorcinol 0.3% serum induced significant improvement in patients with post-laser pigmented lesions, as compared with that of a placebo group8. For melasma patients, 4-n-butylresorcinol 0.3% serum was found to be effective in 84% of the patients with melasma in a 24-week open-treatment study9. In another recent randomized, double-blind, vehicle-controlled, split-face study of 28 patients with melasma, 4-n-butylresorcinol 0.3% serum was found to have significant greater efficacy than a vehicle control after 12 weeks application, based on clinical assessments and colorimetric measurements10. However, a relatively longterm application of 4-n-butylresorcinol was necessary to achieve the hypopigmenting effect in the previous studies. Additionally, adverse events, including mild erythema, dryness, peeling and desquamation, were experienced in 8/28 patients (28.6%) in a recent double-blind study10. Therefore, we designed this clinical study using a lower concentration of 4-n-butylresorcinol.
To the best of our knowledge, this is the first study that has evaluated the effects of 4-n-butylresorcinol 0.1% cream in patients with melasma. Compared to vehicle, the 4-n-butylresorcinol 0.1% cream showed significant efficacy even after 4 weeks of treatment, which lasted until the end of the study.
Overall, the mean MI change at the end of the study for the 4-n-butylresorcinol-treated skin and the vehicle-treated skin were -4.87% and +2.21%, respectively, with a statistically significant difference (p<0.0005). All the patients well tolerated the treatment and the adverse events were mild and transient.
The results showed that a reduced concentration of 4-n-butylresorcinol can be safely used for the treatment of melasma. Moreover, 4-n-butylresorcinol 0.1% enabled equally effective treatment or even more effective treatment in a shorter duration, as compared with that of the previous studies.
However, in this study, we didn't perform clinical assessment of the improvement. Clinical evaluation may not be applicable for evaluating the degree of improvement in those pigmented lesions that are treated by cosmetics, which may show only subtle differences. Bioengineering measurement is the standard method for the evaluation of pigmentary conditions13. Our results were comparable with those by Khemis et al.10 when we compare the objective data obtained by bioengineering measurement. Further studies with diverse methods of bioengineering measurement and clinical evaluation would help to demonstrate the hypopigmenting efficacy of 4-n-butylresorcinol 0.1% cream for the treatment of melasma.
In conclusion, 4-n-butylresorcinol 0.1% cream showed significant efficacy as compared with vehicle alone and it showed good tolerability for the treatment of melasma.
Figures and Tables
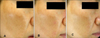 | Fig. 2Patient 1: A 41-year-old woman with melasma was treated with 4-n-butylresorcinol 0.1% cream, and her melanin index (MI) decreased from 221.33 to 210.33 (-11.00). The clinical presentation at baseline with the MI: 221.33 (A), after 4 weeks with the MI: 215.33 (B) and after 8 weeks with the MI: 210.33 (C). |
 | Fig. 3Patient 2: A 46-year-old woman with melasma was treated with 4-n-butylresorcinol 0.1% cream, and her MI decreased from 234.00 to 208.00 (-26.00). The clinical presentation at baseline with the MI: 234.00 (A), after 4 weeks with the MI: 213.00 (B) and after 8 weeks with the MI: 208.00 (C). |
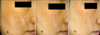 | Fig. 4Patient 3: A 44-year-old woman with melasma was treated with 4-n-butylresorcinol 0.1% cream, and her MI decreased from 223.00 to 189.33 (-33.67). The clinical presentation at baseline with the MI: 223.00 (A), after 4 weeks with the MI: 197.33 (B) and after 8 weeks with the MI: 189.33 (C). |
References
1. Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991. 5:2902–2909.

2. Kobayashi T, Urabe K, Winder A, Jimenez-Cervantes C, Imokawa G, Brewington T, et al. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994. 13:5818–5825.

3. Okubo T, Oyohikawa M, Futaki K, Matsukami M, Fujii A. The inhibitory effects of 4-N-butyl-resorcinol on melanogenesis [abstract]. J Dermatol Sci. 1995. 10:88.
4. Tasaka K, Kamei C, Nakano S, Takeuchi Y, Yamato M. Effects of certain resorcinol derivatives on the tyrosinase activity and the growth of melanoma cells. Methods Find Exp Clin Pharmacol. 1998. 20:99–109.

5. Katagtri T, Okubo T, Oyobikawa M, Futaki K, Shaku M, Kawai M. Novel melanogenic enzymes inhibitor for controlling hyperpigmentation. 1998. In : 20th IFSCC International Congress; 1–11.
6. Shimizu K, Kondo R, Sakai K. Inhibition of tyrosinase by flavonoids, stilbenes and related 4-substituted resorcinols: structure-activity investigations. Planta Med. 2000. 66:11–15.

7. Kim DS, Kim SY, Park SH, Choi YG, Kwon SB, Kim MK, et al. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol Pharm Bull. 2005. 28:2216–2219.

8. Akasaka T, Ohurazaka H, Nishioheda G, Matsumoto S, Takenouchi M. Topically applied 0.3% 4-n-butylresorcinol decreases pigmentation after laser therapy. Environ Dermatol. 2002. 9:11–15.
9. Researching committee of Rucinol(R). The study on the efficacy of Rucinol(R) (4-n-butylresorcinol) in chloasma. Nishinihon J Dermatol. 1999. 61:813–819.
10. Khemis A, Kaiafa A, Queille-Roussel C, Duteil L, Ortonne JP. Evaluation of efficacy and safety of rucinol serum in patients with melasma: a randomized controlled trial. Br J Dermatol. 2007. 156:997–1004.

11. Cassano N, Alessandrini G, Mastrolonardo M, Vena GA. Peeling agents: toxicological and allergological aspects. J Eur Acad Dermatol Venereol. 1999. 13:14–23.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download