Abstract
Porokeratosis is an abnormal disease of keratinization of epidermis. It is clinically characterized by margins covered with keratin layer and it typically has an atrophied macule with a protruded, circular form. Histopathologically, it shows the findings of cornoid lamella. Risk factors for its development include organ transplantation, long-term use of corticosteroids, immunocompromised status, including AIDS, and exposure to ultraviolet light. We herein report a case of atypical porokeratosis in a 38-year-old man who developed porokeratosis involving multiple sites following bone marrow transplantation for myelodysplastic syndrome.
Porokeratosis is a disorder of epidermal keratinization that is inherited as an autosomal dominant trait1. Since it was first described by Mibelli in 1893, it has been classified into 6 types as follows, based on clinical characteristics: porokeratosis of Mibelli, disseminated superficial porokeratosis (DSP), disseminated superficial actinic porokeratosis, porokeratosis palmaris et plantaris disseminata, linear porokeratosis, and punctate porokeratosis1.
In cases in which porokeratosis developed in immunocompromised patients, it is typically seen as a disseminated superficial form which occurs in such multiple sites as the arm, leg and trunk1. To date, many cases have been reported of porokeratosis developing following kidney transplantation. Only 10 cases of prokeratosis developing following bone marrow transplantation (BMT) have been described in the literature, most of which developed following BMT for leukemia. We experienced a case of atypical porokeratosis involving multiple sites, following BMT for myelodysplastic syndrome (MDS).
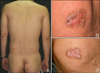
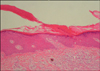
A 38-year-old man presented with an 8-year-history of asymptomatic reddish-brown papules and plaques. The patient was diagnosed with MDS in August 1996, and underwent allogenic BMT following conditioning with busulphan and total body irradiation in February 1997. Two weeks following BMT, cutaneous graft-versus-host disease developed. After treatment with methylprednisolone for 11 months, the lesions resolved completely. The initial dose of methylprednisolone was 125 mg and was tapered throughout the treatment course. Then, without further specific treatment for MDS, the skin lesions reappeared 3 years following BMT. The lesions were multiple, reddish-brown, well-defined papules and plaques 0.5~2.5 cm in size, appearing on both extremities and the trunk (Fig. 1A). Their centers were atrophied and colored pink, and they had raised, brown, keratinized borders (Fig. 1B). The number of lesions increased by 1~2 per year, and the sizes of the lesions also increased. The patient denied a past history of excessive exposure to sunlight. No notable findings were identified in the family history. Physical examination and laboratory tests also revealed no significant findings. Histopathology showed characteristic cornoid lamellae, which appeared as columns of parakeratosis extending at about a 45° angle from a focus of dyskeratotic keratinocytes. There was no granular layer beneath the parakeratotic column (Fig. 2). A biopsy confirmed the diagnosis of porokeratosis. After topical application of 20% urea for several months, the skin lesions showed slight improvement (Fig. 1C).
Immunosuppressive therapy has been reported to exacerbate or initiate the development of porokeratosis. Recently, with the widespread use of organ transplantation and immunosuppressive treatments, the incidence of porokeratosis has increased to 0.34~10.68% of patients who have undergone organ transplantation2. The latency period between organ transplantation and the appearance of porokeratosis ranges from 4 months to 14 years2. Clinically, immunosuppression-associated porokeratosis is more often characterized by multiple rather than single lesions3. Most lesions are found on the legs, arms, and trunk1. Although the exact type of porokeratosis has not always been specified, 50% of reported cases have been identified as DSP3.
Recently, there have been many cases reported with atypical clinical characteristics of porokeratosis. In 1997, Herranz et al.4 reported that most of the cases were the mixed type of porokeratosis of Mibelli, and that DSP developed in 11 patients following kidney transplantation. In our case, the distribution and number of lesions were similar to those of DSP. However, based on the findings of well-defined keratinized lesions that were several centimeters in size and asymmetrical, a diagnosis of porokeratosis of Mibelli could not be ruled out. Therefore, our case was diagnosed as an atypical mixed type of porokeratosis.
Only 10 cases of porokeratosis after BMT have been reported in the English literature, and they are summarized in Table 12,5-9. Most cases of porokeratosis following BMT were associated with leukemia. To date, immunodeficiency disorders associated with porokeratosis have been reported to include AIDS and precancerous blood disorders, as seen in our case3. MDS is a disorder associated with abnormal B cell, NK cell, and CD4 (+) T cell function; therefore this bone marrow stem cell disorder can result in immunodeficiency.
It remains unclear whether the development of porokeratosis in our patient was affected by the immunodeficiencies associated with MDS or by the immunosuppressive treatment associated with the BMT. It is assumed, however, that the pathophysiology of porokeratosis is similar in either case3. To explain this, several hypotheses have been proposed. First, loss of immunosurveillance caused by immunosuppression allows for the proliferation of abnormal keratinocyte clones, which leads to porokeratosis1,3. To support this, Manganoni et al.10 provided evidence that the number and function of Langerhans cells were decreased and the expression of the HLA-DR antigen was reduced in the lesions. It has also been hypothesized that porokeratosis develops because of the higher rates of mitotic division in abnormal clones than in normal keratinocytes.
In most porokeratosis cases, treatment is not necessary. It has also been reported that lesions have spontaneously healed in immunocompromised patients after the primary malignancy was treated. In approximately 7~11.6% of patients, however, porokeratosis is associated with malignancies such as squamous cell carcinoma, basal cell carcinoma, and Bowen's disease. In particular, linear porokeratosis and porokeratosis of Mibelli are highly associated with these malignancies11. Therefore, in immunocompromised patients who develop porokeratosis, the possibility of malignant transformation must be considered, and such cases must be followed by close observation. Histopathology is required to confirm malignant transformation in suspicious cases.
Figures and Tables
References
1. Schamroth JM, Zlotogorski A, Gilead L. Porokeratosis of Mibelli. Overview and review of the literature. Acta Derm Venereol. 1997. 77:207–213.
2. Alexis AF, Busam K, Myskowski PL. Porokeratosis of Mibelli following bone marrow transplantation. Int J Dermatol. 2006. 45:361–365.

3. Levin RM, Heymann WR. Superficial disseminate porokeratosis in a patient with myelodysplastic syndrome. Int J Dermatol. 1999. 38:138–139.

4. Herranz P, Pizarro A, De Lucas R, Robayna MG, Rubio FA, Sanz A, et al. High incidence of porokeratosis in renal transplant recipients. Br J Dermatol. 1997. 136:176–179.

5. Lederman JS, Sober AJ, Lederman GS. Immunosuppression: a cause of porokeratosis? J Am Acad Dermatol. 1985. 13:75–79.

6. Rio B, Magana C, Le Tourneau A, Bachmeyer C, Levy V, Hamont N, et al. Disseminated superficial porokeratosis after autologous bone marrow transplantation. Bone Marrow Transplant. 1997. 19:77–79.

7. Gilead L, Guberman D, Zlotogorski A, Vardy DA, Klaus SN. Immunosuppression-induced porokeratosis of Mibelli: complete regression of lesions upon cessation of immunosuppressive therapy. J Eur Acad Dermatol Venereol. 1995. 5:170–172.

8. Raychaudhuri SP, Smoller BR. Porokeratosis in immunosuppressed and nonimmunosuppressed patients. Int J Dermatol. 1992. 31:781–782.

9. Ghigliotti G, Nigro A, Gambini C, Farris A, Burroni A, de Marchi R. Mibelli's porokeratosis after bone marrow transplantation. Ann Dermatol Venereol. 1992. 119:968–970.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download