INTRODUCTION
Hematodermic neoplasm (HN) is a clinically aggressive neoplasm with a high incidence of cutaneous involvement and risk of leukemic dissemination. This entity was initially believed to arise from natural killer (NK) cells with CD56 expression, and was categorized as blastic NK lymphoma in the WHO classification1. However, in the recent WHO-EORTC classification of cutaneous lymphomas, the term blastic NK cell lymphoma has been replaced with CD4+/CD56+ HN because of its derivation from a plasmacytoid dendritic cell precursor2. CD123+ neoplasms may be derived from plasmacytoid type 2 dendritic cell precursors and share common myeloid/NK precursor stem cells3.
Few cases of CD4-CD56+ HN are reported in the literature, and they show clinical features of CD4+CD56+ HN4,5. The terminology of CD4+CD56+ HN may therefore cause confusion in diagnosing NK cell neoplasms. Herein we report an unusual case of 'CD4-/CD56+/CD123+ HN' showing aggressive behavior with initial presentation in the skin and subsequent multiple metastases.
CASE REPORT
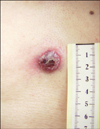
A 68-year-old Korean man was admitted with a rapidly growing, 1.9×1.8 cm exophytic erythematous round plaque on the parasternal area (Fig. 1). This asymptomatic skin lesion had abruptly appeared 3 months before his first visit. On palpation, the liver was enlarged about 3 fingers' breadth under the right costal border, but the axillary and inguinal lymph nodes were not palpable.
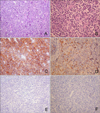
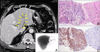
A skin biopsy revealed a diffuse heavy infiltration of similar medium-sized lymphoid cells in the entire dermis that extended into the superficial muscular layers but sparing the epidermis. These cells had medium to large nuclei with fine chromatin, small nucleoli, and scanty cytoplasm, and resembled lymphoblasts without any angiocentricity or necrosis (Fig. 2A, B). Immunohistochemistry revealed strong positives for CD56 (Fig. 2C), CD123 (Fig. 2D), and CD45RA but negativities for sCD3, CD8, CD4 (Fig. 2E), CD 68 (Fig. 2F), CD13, CD15, CD20, CD34, CD45RO, TdT, and myeloperoxidase. Epstein Barr virus was not detected in in situ hybridization using EBER, and TCR-rearrangement showed germline configuration. Atypical cells were not found in bone marrow biopsy and peripheral blood smears. A liver scan and abdominal ultrasound showed hepatosplenomegaly, thromboses in the left and main portal veins, and a 10 cm heterogeneous hypoechoic mass in the left lateral segment of the liver. Multiple lymph nodes were also enlarged along the porta hepatis on abdominal CT scan (Fig. 3A). Ultrasound-guided fine needle biopsy revealed a well-demarcated, discrete infiltration of atypical lymphoid cells with replacement of the normal hepatocytes (Fig. 3B). Immunohistochemistry showed strong positives for CD56 and CD45RA; the other markers were negative in the liver tissues (Fig. 3C). With a diagnosis of CD4-/CD56+/CD123+ HN with liver metastasis, the patient underwent hyper-CVAD chemotherapy with complete remission, but bone pain on the right humerus and diplopia developed 6 months after chemotherapy. We cytologically confirmed bone and central nervous system metastases by biopsies. He died 13 months after initial diagnosis during conservative treatment for pulmonary edema and renal failure.
DISCUSSION
Up to 90% of HN patients have asymptomatic solitary or multifocal skin lesions such as papules, nodules, or bruise-like lesions, and bone marrow involvement with or without concurrent extracutaneous localizations with a fatal course1,6. The diagnosis is based on skin biopsy, which shows diffuse infiltrations of medium-sized blastic cells in the dermis. In the leukemic phase, intermediate-sized primitive cells with blastlike morphology are present in blood and bone marrow, similar to those found in skin lesions2.
Typical cases of HN that completely lack CD4 or CD56 expression represents a diagnostic problem, as only a few CD4-cases are genuine HN5,7. Ascani et al.4 suggested that lack of CD4 expression in HN may be explained either by genuine phenotypic variation in this neoplasm or by low levels of CD4 within neoplastic cells. In CD4 negative HN, the identification of plasmacytoid dendritic cell lineage is more critical for diagnosis than CD4 expression itself. Usually, this neoplasm has eccentric nuclei with small-to-medium, pale, agranular eosinophilic cytoplasm, and expresses markers of plasmacytoid dendritic cells such as CD123, TCL1, and BDCA-2. Additionally, neoplastic cells are positive for CD4, CD56, and CD43, with variable staining for CD68 and TdT. Cutaneous lymphocyte-associated antigen are strongly reactive in plasmacytoid dendritic cells3, but the role of CD4 positivity over other T cell related antigens such as CD2, sCD3, CD5, CD7, and CD45RO, which are usually negativitie8-11, is unclear. We observed clinical and histopathological features of HN, with CD123+ but CD4-. CD123 expression may be a useful clue for the diagnosis of rare neoplasms.
Extracutaneous involvement included bone marrow (13/20), lymph nodes (8/20), blood (4/20), and palate (2/20)12. HN has a highly aggressive clinical course with a poor prognosis and a median survival of 14 months2,13. Most courses are short and aggressive, often terminating in fulminant leukemia and death1.
Acute myeloid leukemia with myelomonocytic differentiation in the skin is difficult to differentiate from HN, especially as the lesions also express CD4, CD56, CD43, and CD68, but myeloperoxidase is usually positive, in contrast to HN14. Primary and secondary cutaneous NK/T-cell lymphomas closely resemble HN, but show angiotropism with necrosis, cytoplasmic positivity for Giemsa, and CD4 expression with CD4 negativity. Epstein-Barr virus association in NK/T-cell lymphomas can also differentiate them from HN2.
The frontline therapies for HN, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) and hyper-CVAD, generally produce a complete clinical response. We initiated hyper-CVAD treatment and then switched to high dose methotrexate/cytarabine. His disease relapsed with bone and brain disease 6 months after clinical complete remission. The early liver involvement indicated rapid progression such as metastases to the bone and brain. Radioisotope liver scans and ultrasound-guided fine needle aspiration biopsy are important diagnostic measures for staging aggressive courses and multi-organ metastasis.
In conclusion, our case emphasizes that CD4-negative cases of HN exist. Although HN is a distinct entity based on clinicopathological features, variable expression of T-cell related antigens such as CD2, sCD3, CD5, CD7, and CD45RO can confuse diagnosis of this neoplasm15. Cytogenetic studies such as comparative genomic hybridization are required for this heterogenous entity, as for subcutaneous panniculitis T cell lymphoma and primary cutaneous large B cell lymphoma, and to elucidate genetic mechanisms of lymphoma development and progression16,17.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download