Abstract
Background
Acute generalized exanthematous pustulosis (AGEP) is a rare and severe subtype of drug eruption, characterized by acute, extensive, non-follicular, sterile pustules on an erythematous background, accompanied by fever and leukocytosis.
Objective
The purpose of this study was to characterize AGEP in Korean patients in terms of clinical, laboratory, and pathologic findings.
Methods
Thirty-six patients (M:F=17:19) with AGEP were identified from an extensive review of medical records over a 15 year period. All patient cases were confirmed by biopsy and fulfilled the diagnostic criteria.
Results
The patient ages ranged from 4~80 years (37.6±19.4). The incubation period was 1~23 days. The duration of disease was 5~14 days. Neutrophilia (36/36), high CRP (14/36), and eosinophilia (30/36) were common laboratory findings. A history of drug administration existed in 23 of 36 patients; herbal medications, lacquers and radiocontrast media were the unique causative drugs. Spongioform subcorneal or intraepidermal pustules in the epidermis was observed in all patients. Thirty-six patients were subdivided into 2 groups: group A (n=23) was strongly associated with known agents; and group B (n=13) had no identified causative agents. There was no significant difference between the 2 groups.
In 1968 Baker and Ryan1 identified 5 patients with drug-related pustular eruptions of an acute course, who had no history of psoriasis. The term "acute generalized exanthematous pustulosis" (AGEP) was first introduced by Beylot et al.2 in 1980. Subsequently, AGEP was better characterized by Roujeau et al.3 and Chang et al.4.
AGEP is now recognized as a disease entity that is distinct from pustular psoriasis. AGEP has 3 characteristic features: 1) an acute generalized formation of numerous, non-follicular, intraepidermal or subcorneal sterile pustules (<5 mm) on an extensive erythematous background, in the absence of bacterial infection, especially on the main flexoral folds as well as on the other parts of the body and face; 2) neutrophils appear after T cell infiltration; and 3) the possibility of inducing the dermatologic reaction by patch testing with the corresponding drug. A few cases related to viral infections5, dietary supplements, hypersensitivity to mercury, radiation3, and spider bites6 have been reported, but 90% of cases of AGEP are attributed to systemic drugs, especially antibiotics, such as amino-penicillin and macrolides4. It has been hypothesized that spider venom or viral infection such as Coxsackie B4, cytomegalovirus and parvovirus B19 may induce inflammatory cytokines (Interleukin 8 or granulocyte-macrophage colony stimulating factor (GM-CSF)) and trigger AGEP. Cutaneous symptoms develop quickly (within a few hours) and resolve rapidly (within a few days) without treatment. Systemic symptoms and signs, such as fever and leukocytosis, are present. Mucous membrane involvement occurs in about 20% of cases, and is usually restricted to a single area (oral mucosa)7.
To date, there have been only limited case series of AGEP in the Korean literature. Kim et al.8 first reviewed 8 cases of pustular drug eruptions, and recently Lim et al.9 reported 9 cases of AGEP compared to pustular psoriasis. In the current series, we review the clinicopathological characteristics of 36 patients with AGEP who had been diagnosed in 4 hospitals in Korea between 1994 and 2008. Our report is the second largest study on the clinicopathological features of AGEP performed, according to the recent criteria and scoring system (the AGEP validation score of EuroSCAR)2-4,7.
Data were collected retrospectively from the medical records of 124 patients who had been clinically diagnosed or suspected to have AGEP, between January 1994 and July 2008, in the Departments of Dermatology of 4 medical centers in Korea. We reviewed the biopsy slides, laboratory findings, treatment regimens, and medical and family histories of all patients. Among 124 patients surveyed, 88 who lacked biopsy-proven lesions or who did not have an adequate amount of clinical or laboratory data were excluded, thereby leaving 36 enrolled in this study. All cases fulfilled the criteria according to the AGEP scoring system of the EuroSCAR study group7. The AGEP validation score (EuroSCAR group criteria) was suggested after a multinational European study (EuroSCAR) which included 97 validated cases of AGEP. The AGEP validation score is a standardized scoring system and is based on clinical features and histopathology.
Thirty-six patients were subdivided into 2 groups, according to the presence or absence of causative agents. Twenty-three patients belonged to the group strongly associated with systemic drugs and 13 patients had no known causative drugs. The 2 groups were compared in terms of age, gender, systemic symptoms (such as fever, myalgias, or headaches), duration of disease, history of drug allergy, laboratory data, and pathologic findings.
Results were analyzed with a statistical package (SAS System for Windows, version 9.1; SAS Institute Inc, Cary, NC, USA). To compare continuous data such as age, duration of disease, and EuroSCAR between the 2 groups, the Wilcoxon rank sum test was used. The Fisher's exact test was performed to compare gender, systemic symptoms, drug allergy history, neutrophilia, and treatment regimens between the 2 groups. A p-value <0.05 was defined as statistically significant.
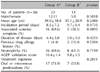
Table 1 demonstrates the general characteristics of 36 patients with AGEP. The patient ages ranged from 4~80 years (mean, 37.6±19.4 years). Females were slightly predominant (F:M=19:17). Three of the 36 patients had a drug allergy history, and 1 patient had a history of psoriasis. Our patients had a number of other systemic diseases, including gastric ulcers, asthma, diabetes mellitus, hypertension, bronchitis, Sjogren's syndrome, urinary tract infections, multiple sclerosis, and malignancies. One patient was a chronic hepatitis B carrier. Two patients gave birth 2 days before eruption of AGEP. The incubation period was between 1 and 23 days (mean, 9.5±6.2 days). The duration of disease was between 5 and 14 days (mean 7.61±2.97). Numerous, non-follicular, sterile pustules on an extensive erythematous background demonstrated in all patients and the lesions resolved with or without exfoliations (Fig. 1, 2, 3). Associated systemic symptoms, such as fever, myalgias, or headaches were noted in 21 of 36 patients. None of the patients had mucosal involvement. The AGEP validation score was between 8 to 12 days (mean 10.2±1.5).
Neutrophilia (11,521±5,603/µl; normal <8×103/µl; 36/36), elevated CRP (66.5±57.1 mg/dl; normal <0.47 mg/dl; 100% of available patients), and eosinophilia (733.2±902.5/µl; normal <7×102/µl; 30/36) were commonly detected. Renal (creatinine 1.0±0.2 mg/dl; normal <1.2 mg/dl; 8/36) and liver function tests (aspartate aminotransferase 28±17 U/L; normal <40 U/L; 11/36; and alanine aminotrasnferase, 33±25 U/L; normal 40 U/L; 10/36) were generally within normal limits, except for the 2 patients with hepatocellular carcinoma.
Systemic drugs were the most common etiology (23/36 [63.8%]) for AGEP, especially the antibiotics. Among the remaining patients, offending factors included herbal medications (n=4), lacquers (n=3), analgesics (n=2), radiocontrast media (iopamidol; n=2), chemotherapeutic agents (n=1), clenbeterol (n=1), clopidogrel (n=1), and antihistamines (n=1). Thirteen patients had no known contributory causes.
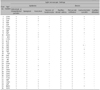
When 36 patients were subdivided into group A (strongly associated with known possible agents) and group B (no identified causative agents), there was no difference between the 2 groups with respect to gender, age, incubation period, duration of disease, associated systemic symptoms, drug allergy history, neutrophilia, AGEP validation score, and treatment regimens (Table 2).
Spongioform subcorneal or intraepidermal pustules in the epidermis were observed in all patients, as well as exocytosis (17/36) and necrosis of keratinocytes (2/36) (Fig. 4). Psoriatic changes, such as acanthosis and papillomatosis, were absent in all patients. In the dermis, papillary edema and perivascular infiltrates of neutrophils or eosinophils were seen in all patients. Leukocytoclastic vasculitis was observed in 11 patients (Table 3).
Treatment regimens varied, as follows: 33 patients were treated with systemic agents (24 patients with oral or intravenous prednisolone, and 9 patients with oral antihistamines). One patient was treated with topical steroids and 2 patients were not treated.
In this study, a history of drug administration existed in 23 of 36 patients (63.8%), especially the antibiotics such as amoxicillin and hydroxychloroquine. Among 8 cases of AGEP described by Kim et al.8 and 9 cases of AGEP described by Lim et al.9, the causative agents of AGEP were antibiotics and analgesics, except in one case (herb medication). The recent large scale multinational case-control study (the EuroSCAR study) revealed highly suspected drugs for causing AGEP, including pretinomycin, ampicillin/amoxicillin, quinolones, (hydroxyl) chloroquine, antiinfective sulfomides, terbinafine and diltiazem7. The association with systemic drugs that we found was lower than in previous reports2-4. One possible reason for this is that the skin patch test to identify causative drugs rarely elicits a cutaneous reaction. The expense of skin patch tests can also burden patients. There are also other possible reasons: in East Asia, especially Korea, alternative or complementary medicine is universal and accessible. The patients are likely to be unknowingly exposed to multiple, various drugs or ingredients, thus, it is difficult for clinicians to perform the skin patch test because patients cannot remember the exact medications preceding the eruptions of AGEP. As well, the specific drug ingredients are not easy to identify if they are not prescribed by the clinics.
A past history of psoriasis was evident in only one patient. In the Korean dermatologic literature, Lim et al.9 reported 2 AGEP patients among the 9 in their study as having a family history of psoriasis. The fact that a history of psoriasis was found to be unrelated to AGEP in our patients can be a useful diagnostic clue.
In this study, there was no statistically significant difference between group A, which was strongly associated with known possible agents, and group B, with no known causative agents; the 2 groups were basically identical in these terms. In light of this fact, drugs are the most likely precipitant of AGEP in the group without identified causative drugs. The causative drugs that bring about AGEP in the group without identified causative drugs may be administered to the body as health food or other possible vehicles. Conversely, Chang et al.4 suggested that the reason for the low association with systemic drugs may be due to a specific feature of Asian people, for example a physiological difference and that the drug may be of less value as an etiology of AGEP than previously thought. This conclusion is more aligned with the fact that a difference in the age of onset was observed between group A, which was strongly associated with known possible agents and group B, without an identified causative agent; in these terms it is suggested that the groups are different. Further, large retrospective studies are needed to clarify the relationship between drugs and AGEP in Asia.
It was interesting in our findings to note that herbal medications, lacquers, and radio-contrast media were suspicious for one of the major offending factors of AGEP. An increasing trend has been observed in the use of alternative and complementary medicines, such as herbal medications. Herbal medications are an easily accessible remedy compared to conventional therapy in Korea, and with less expense. It is not surprising that herbal medications can cause AGEP because herbal medications are known to contain unsafe levels of heavy metals (including mercury)10, synthetic drugs, or contamination with microorganisms11. Because AGEP is self-limited, more patients with AGEP due to herbal medication were thought to exist.
Lacquers are commonly used for decorating or protecting furniture, floors, and ornaments in East Asia. Interestingly, chicken broth containing lacquers has been considered to be an alternative medicine for peptic ulcers and as a health food to prevent certain hypersensitivity reactions to lacquers in Korea. Park et al.12 already reported a series of cases of AGEP induced by lacquer chicken in Korean patients. Likewise, all of our patients developed AGEP shortly after ingestion of lacquer as lacquer chicken broth, and then the problem resolved soon after treatment. They had no history of drug ingestion, recent infections, or contact with mercury. Adverse reactions to radiocontrast media are common, estimated to occur in 13% of subjects receiving iodinated media13. Typical cutaneous eruptions of AGEP commonly occurred soon after administration of radiocontrast media (iopamidol) in 2 of our patients. They had no other history of drug ingestion, recent infections, or contact with mercury, and they had not taken any radiocontrast media during computerized tomography exams. Therefore, we suspect that the radiocontrast media was the trigger for AGEP in 2 patients. Though it is rare, there being only a few cases of AGEP caused by radiocontrast media reported14-17. More case reports are expected because of the recently increasing use of radiocontrast media.
We suggest that the causative drugs may be still the main precipitant of AGEP in this study of 36 Korean patients. Antibiotics, herbal medications, lacquers, and radiocontrast media are suspected to be the newly rising factors. It is our hope that dermatologists will not hesitate to diagnose AGEP even though the suspected causative drugs are not completely identified or proven.
Figures and Tables
Fig. 1
Multiple, pin-head sized, non-follicular pustules with an erythematous base on the trunk in case 36 (A) and the big folds of lower extremities in case 10 (B).
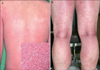
Fig. 4
(A) Spongioform subcorneal pustules consisted of polymorphonuclear leukocytes in the epidermis (H&E, ×100, case 7). (B) Leukocytoclastic vasculitis in the dermis (H&E, ×100, case 1).

References
1. Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968. 80:771–793.
2. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases). Ann Dermatol Venereol. 1980. 107:37–48.
3. Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991. 127:1333–1338.

4. Chang SL, Huang YH, Yang CH, Hu S, Hong HS. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008. 88:363–365.
5. Rouchouse B, Bonnefoy M, Pallot B, Jacquelin L, Dimoux-Dime G, Claudy AL. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986. 173:180–184.

6. Davidovici BB, Pavel D, Cagnano E, Rozenman D, Halevy S. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006. 55:525–529.

7. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007. 157:989–996.

8. Kim SJ, Lee SH, Ahn SK, Lee WS, Lee BJ. Clinicopathologic study of pustular drug eruption. Korean J Dermatol. 1994. 32:554–561.
9. Lim JY, Jang HS, Oh CK, Kwon KS, Kim MB. Clinicopathologic study of generalized pustular psoriasis and acute generalized exanthematous pustulosis. Korean J Dermatol. 2002. 40:244–252.
10. Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy metal content of ayurvedic herbal medicine products. JAMA. 2004. 292:2868–2873.

11. Bogusz MJ, al Tufail M, Hassan H. How natural are 'natural herbal remedies'? A Saudi perspective. Adverse Drug React Toxicol Rev. 2002. 21:219–229.
12. Park YM, Park JG, Kang H, Houh D, Byun DG, Kim JW. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000. 143:230–232.

13. Foti C, Bonamonte D, Conserva A, Antelmi AR, Antonaci CE, Angelini G. Occupational allergic contact dermatitis to a non-ionic iodinated contrast medium containing iomeprol. Contact Dermatitis. 2008. 59:252–253.

14. Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003. 30:723–726.

15. Peterson A, Katzberg RW, Fung MA, Wootton-Gorges SL, Dager W. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol. 2006. 187:W198–W201.

16. Belgodere X, Wolkenstein P, Pastor MJ. Acute generalized exanthematous pustulosis induced by iopamidol. Ann Dermatol Venereol. 2004. 131:831–832.
17. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009. 145:683–687.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download