Abstract
Background
Systemic contact dermatitis commonly occurs with the intake of rhus (boiled chicken with rhus) as a health food and a folk medicine to cure gastrointestinal diseases in Korea. Rhus companies insist they have the technology for rhus detoxification. However, the numbers of systemic allergic contact dermatitis patients, caused by rhus, have not decreased. The principle of present techniques for rhus detoxification is the induction of the polymerization of urushiol, but polymerized urushiol may still have antigenicity, although to a diminished degree. The Korean Food and Drug Administration (KFDA) has a regulation to control urushiol use as a food. However, the laboratory method that KFDA uses for detection of rhus can only detect the urushiol monomer.
Objective
We conducted experiments to detect polymerized urushiol in rhus products, which were considered not to include urushiol by the KFDA.
Methods
Rhus product approved by the KFDA was separated with chloroform. The chloroform fractionation was accomplished on a recycle HPLC system. Four peaks were achieved and evaporated to give an amorphous powder. Each powder was analyzed on a NMR system and mass spectrometer.
Systemic contact dermatitis commonly occurs with the intake of lacquer (rhus) in Korea, because rhus lacquer is thought to be effective for the treatment of gastrointestinal problems and good for health1-3. Recently, rhus products have been commercialized due to a national project of the Ministry of Agriculture and Forestry of Korea, and also, manufacturers have insisted that they possess proprietary technology for rhus poison detoxification. However, there have been many people visiting dermatologic clinics who are suffering from the ingestion of rhus products. Urushiol, the sap of the Japanese lacquer tree, is the active immunogen of rhus. Urushiol, acting as hapten, binds with self-protein to then generate neo-antigenic determinants4,5. The principle of current rhus poison detoxification techniques is the elimination of urushiol. Current methods that induce the oxidation and the polymerization of urushiol include heating, boiling with botanical materials or high protein foods, as well as a process that uses an oxidizing agent or enzyme6. The process diminishes the allergenicity of urushiol through reduction of an effector, but polymerized urushiol still has antigenicity - although to a diminished degree6,7. The Korean Food and Drug Administration (KFDA) has regulations to control urushiol use as a food. However, the KFDA laboratory method for urushiol detection can detect only the rhus monomer. Accordingly, present rhus products that were considered not to include urushiol by KFDA possibly do contain polymerized urushiol. Therefore, we conducted experiments for detection of polymerized urushiol in rhus products approved by the KFDA.
Commercially available rhus product approved by the KFDA was separated with chloroform, then the chloroform fraction was evaporated to give a yellow powder. The powder was dissolved in chloroform at the concentration of 2.5 mg/ml, and then fractionated on a recycle HPLC LC-908 system (Japan Analytical Co. Ltd., Tokyo, Japan) after filtration through a 0.45 um syringe filter. Recycle HPLC was performed on a column (JAIGEL-2H). Mobile phase was an isocratic system of chloroform. The flow rate was 3.5 ml/min, and the injection volume was 2 ml. The eluent was detected by RI. Four peaks were collected according to retention time, and individual peaks were evaporated to give a powder, named A~D.
Four kinds of amorphous powder (A~D) from the recycle HPLC peaks were analyzed on a mass spectrometer. All mass spectra were acquired on a hybrid, ion-trap, time-of-flight mass spectrometer (Shimadzu LCMS-IT-TOF, Kyoto, Japan) equipped with an ESI source (ESI-IT-TOFMS) in negative ion mode at a mass resolution of 10,000 full-width at half maximum (FWHM). Accurate masses were corrected by calibration using the sodium trifloroacetate clusters as internal references. The analytical conditions were as follows: scan range, m/z 100~1,000; positive spray voltage, +3.0 kV; detector voltage, 1.65 kV; skimmer voltage, 9.0 V; pressure of TOF region, 1.5×10-4 Pa and temperature, 408℃; ion source temperature, 2,008℃; trap cooling gas (Ar) flow rate, 94 ml/min; ion trap pressure, 1.8×10-2 Pa; collision gas (Ar) flow rate, 43 ml/min; ion accumulation time, 10 ms; precursor ion selected width, 3.0 m/z units, and the selected time, 20 ms. MS data was acquired by using the data-dependent function. Approximate sample solutions were prepared by dissolving each sample in a solution of MeOH to a final concentration of 50 ug/ul. All ions produced were finally introduced into the TOF instrument for accurate mass determinations. Data acquisition and analysis were performed with LC Solution 3.0 software (Shimadzu, Kyoto, Japan). The Shimadzu Composition Formula Predictor was also used to verify identifications.
Four kinds of powder (A~D) from recycle HPLC peaks were analyzed on a 1H NMR system. All NMR spectra were acquired on a Bruker AVANCE 500 spectrometer equipped with a cryo triple-resonance probe with an actively shielded pulsed field gradient (PFG) coil. Chemical shifts for 1H are reported relative to DMSO solvent peak at 2.5 ppm. All experiments were performed at 298 K. The 1H spectra were acquired with 16 K data points, 8,012 Hz for spectra acquired in DMSO. The repetition delays were set to 2 sec, and 64 scans were averaged for each FID. All NMR data were processed using TopSpin (Bruker).
Four different fractions (A~D) were analyzed with a mass spectrometer. Result of the fraction C is shown in Fig. 1. A peak corresponding to molecular weight 638 could be identified.
Four different fractions (A~D) were analyzed with 1H NMR. Result of the fraction C is shown in Fig. 2. The existence of a phenol ring originated from catechol could be identified with the signals between 7 and 7.5 ppm; that of CH2 with signals between 1 and 1.5 ppm, and that of CH3 with signal at 0.8 ppm could be identified.
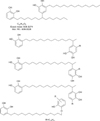
With these 2 analyses, the existence of a substance suspected to be urushiol dimer with a molecular weight 638, can be drawn (Fig. 3).
Many Koreans consume rhus extract (Japanese lacquer tree, Rhus verniciflua) in various forms such as lacquer with boiled chicken, duck and rabbit, a lacquer sap drink, or via inhalation, relying on folk medicine beliefs that rhus is good for the health. For that reason, systemic contact dermatitis caused by rhus occurs frequently in Korea1-3. Adverse effects, due to ingestion of this rhus, have been significant. Yun et al.8 reported that 32% of the people who ingested boiled chicken with rhus, for the first time, exhibited a rash. Won et al.3 conducted a clinical study of 147 patients with systemic contact dermatitis from ingestion of boiled chicken with rhus. They reported that when people ingested boiled chicken with rhus, 1 out of 4 exhibited systemic contact dermatitis. Erythematous maculopapules, erythroderma and erythema multiforme-like lesions were among the cutaneous manifestations: 50.6%, 40.9% and 8.4%, respectively. They also reported that leukocytosis and abnormal liver function were identified in 61.2% and 16.3% of those who exhibited cutaneous manifestations, respectively. Simultaneously, systemic symp toms such as pyrexia, rigor, headache, chest tightness and dyspnea have also been reported1,2,8,9.
Urushiol, an antigen of rhus, acts as a hapten and exhibits antigenicity, binding with self-protein4,5. Urushiol is a catechol complex that is comprised of either a C15 or C17-alkyl or alkenyl group on the side chain. Depending on side chain type, the complex can consist of 10 or more slightly different similar structures. Both saturated and unsaturated side chains were identified1,6,10,11. Urushiol is stabilized by inducing oxidation and polymerization with laccase (p-diphenol oxidase enzyme) under appropriate humidity1,6. Its antigenicity decreases through this stabilization process, but does not completely disappear, and may cause an immune reaction7.
Rhus detoxification means removing urushiol. Currently, available rhus detoxification is accomplished through simple heating, methods involving oxidizing agents or enzymes, methods utilizing plant materials such as medicinal herbs, or heating with protein6. Urushiol reacts with self-protein at the catechol phenol ring and with unsaturated side chain sites, which are the primary active sites for oxidation and polymerization10. Accordingly, reactivity differs depending on the extent of unsaturation on the side chains. Therefore, urushiol, with saturated side chains, reacts in less than 50% of the subjects; whereas, urushiol with unsaturated side chains reacts in more than 90%10. Simple heating, methods accompanying oxidizing agents or enzymes, and methods accompanying heating with protein use these characteristics, and induce generation of urushiol polymers through oxidation and polymerization of the urushiol catechol phenol ring and unsaturated side chains. In this way, reactivity of urushiol is mitigated, reducing active sites that bind with self-protein6,10,12. However, as active sites that bind with self-protein can remain within a urushiol polymer, too, antigenicity does not completely disappear. So, if it is exposed to those who are already sensitized, symptoms such as contact dermatitis can be induced6,7,10.
Now, the KFDA has appointed rhus as an 'ingredient that cannot be used in the food', and the standardized laboratory test method for urushiol identification as a high performance liquid chromatography (HPLC)-UV absorption detector with a gas chromatography-mass spectrometer. In case of rhus-related foods, the KFDA approves a food product if urushiol is not detected in accordance with this norm. However, these test methods use samples pretreated several times with a filtration of acetone, hexane and acetonitrile as solvents, so that the urushiol polymer is filtered and not detected. In addition, as only the presence of 4 types of urushiol monomer peaks (C15monoene, diene, triene, saturated) is determined, urushiol polymer cannot be identified6. In other words, present rhus products, which were considered not to include urushiol potentially by KFDA, potentially contain urushiol polymer, in which antigenicity still remains.
For this reason, in order to identify the existence of polymers, we prepared samples fractionated with chloroform solvent - which can dissolve polymers - utilizing one of the commercially available rhus extract products that were considered not to include urushiol by the KFDA. Then, we used a method to obtain fractions with a recycle preparative HPLC system. Also, we carried out 1H NMR analysis using DMSO-d6 as a solvent, which can dissolve polymers of high molecular weight. As a result, a substance with molecular weight 638 that is suspected to be urushiol dimer was identified. This means that urushiol polymers - such as dimmers - in which antigenicity potentially remains, exist in the rhus products approved by the KFDA. So, if these rhus products are exposed to those who are already sensitized, adverse effects, such as contact dermatitis, may present. Even if the antigenicity of this rhus product is low, the product is not completely detoxified.
The present experiments have some limitations. First, we used only one rhus extract product approved by the KFDA as a sample. So, a reproducibility test, which checks if the same results from experiments with other products will be obtained or not, should be carried out. Secondly, some impurities might exist in the samples as a result of simplifying filtration processes in order to identify urushiol polymers. These impurities may be interfering in the precise mass spectrometry and structure analysis. Thirdly, structure analyses using 2-dimensional or 3-dimensional NMR, in addition to 1H NMR, were not conducted.
In this study, we identified a substance, suspected to be urushiol dimer, present in one of the commercially available rhus products. Therefore, even if the antigenicity of the rhus products is low, the products may cause adverse effects, such as skin eruptions, due to inomplete detoxification. Thus, imprudent public relations and distribution of potentially unsafe rhus products should be banned; efforts to enhance the approval standards for the distribution of rhus-related products are required.
Figures and Tables
ACKNOWLEDGMENT
We would like to thank Gil Ja Jun, Sung Uk Chae, Ho kyoung Kim, Eun Hee Kim and Kun Cho for their work in conducting experiments.
References
1. Park SD. Herb medicine-induced adverse effects in dermatological field. J Korean Med Assoc. 2005. 48:325–332.

2. Park SD, Lee SW, Chun JH, Cha SH. Clinical features of 31 patients with systemic contact dermatitis due to the ingestion of Rhus (lacquer). Br J Dermatol. 2000. 142:937–942.

3. Won TH, Seo PS, Park SD, Kim DL, Park JH. Clinical features in 147 patients with systemic contact dermatitis due to the ingestion of chicken boiled with Japanease lacquer tree. Korean J Dermatol. 2008. 46:761–768.
4. Lopez CB, Kalergis AM, Becker MI, Garbarino JA, De Ioannes AE. CD8+ T cells are the effectors of the contact dermatitis induced by urushiol in mice and are regulated by CD4+ T cells. Int Arch Allergy Immunol. 1998. 117:194–201.

5. Kalish RS, Wood JA, LaPorte A. Processing of urushiol (poison ivy) hapten by both endogenous and exogenous pathways for presentation to T cells in vitro. J Clin Invest. 1994. 93:2039–2047.

6. Choi HS, Kim MK, Park HS, Yun SE, Mun SP, Kim JS, et al. Biological detoxification of lacquer tree (Rhus verniciflua stokes) stem bark by mushroom species. Food Sci Biotechnol. 2007. 16:935–942.
8. Yun SK, Ko KB, Song IM, Choi SP, Ihm CW. Epidemiologic study on systemic contact dermatitis due to ingestion of rhus. Korean J Dermatol. 2002. 40:253–257.
9. Oh SH, Haw CR, Lee MH. Clinical and immunologic features of systemic contact dermatitis from ingestion of Rhus (Toxicodendron). Contact Dermatitis. 2003. 48:251–254.

10. Xia Z, Miyakoshi T, Yoshida T. Lipoxygenase-catalyzed polymerization of phenolic lipids suggests a new mechanism for allergic contact dermatitis induced by urushiol and its analogs. Biochem Biophys Res Commun. 2004. 315:704–709.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download