Abstract
Background
Over the past decade, combination therapies have become a mainstay of dermatologic care in psoriasis. Combination therapies are often more effective and safer than large dose single-agent therapies. With the emergence of new biologic therapies, dermatologists now have a wider array of tools to treat psoriasis. Although much data exists regarding cyclosporine or biologic agents alone for psoriasis, little is known about the efficacy, safety and tolerability of combination regimens.
Objective
We designed a study to evaluate the efficacy and safety of etanercept and cyclosporin combination therapy in patients with refractory psoriasis.
Methods
We administered oral cyclosporine (200 mg daily) and subcutaneous etanercept 50 mg weekly injections until symptoms improved, then maintained treatment at a reduced dose. Seven patients with refractory psoriasis were evaluated 4 weekly.
Results
All 7 patients showed rapid responses to combination therapy. Mean Psoriasis Area and Severity Index reductions following conditioning therapy (mean: 6.85 weeks) and maintenance therapy (mean: 56.5 weeks) were 94.9% and 93.2%, respectively.
Conclusion
Etanercept and low-dose cyclosporine combination therapy appears to be a safe and efficacious alternative treatment strategy for patients with refractory psoriasis. The combination induced rapid improvement in patients with refractory psoriasis and dramatically improved their quality of life. Clinical studies including larger patient cohort are required to validate the safety and efficacy of this combination therapy.
Psoriasis is a chronic inflammatory skin disorder in which both adaptive and innate immunities play important roles. It affects 1~3% of the population worldwide1. Traditionally, psoriasis therapies involve phototherapy and systemic therapies, such as methotrexate, acitretin and cyclosporine. The treatment of moderate to severe psoriasis has been limited by the agents' safety and efficacy, which means that treatment needs to be alternated or discontinued at intervals to avoid potential toxicity. Recently, more targeted therapies have emerged with apparently fewer toxic side effects as understanding of the immunopathology of psoriasis improve, such as biologic agents, which act on precise targets in the immunologic cascade to control psoriasis. Nevertheless, severe psoriasis can still be extremely difficult to treat in some patients, even with biological therapies. Therefore, there is a need for novel alternative therapies for refractory psoriasis.
Etanercept is a recombinant TNF-α receptor fusion protein consisted of 2 extracellular ligand-binding domains of the human p75 TNF-α receptor fused to the Fc portion of human IgG1. It has received FDA approval for the treatment of both psoriasis and rheumatoid arthritis. A double-blinded, placebo-controlled, multi-center study showed that, by 24 weeks, 56% of etanercept-treated patients showed Psoriasis Area and Severity Index (PASI) improvements of at least 75%2. However, the high cost of this treatment remains an issue for many patients.
Cyclosporine works through inhibition of cell-mediated immune responses, specifically T lymphocyte function, by decreasing production of IL-23. Cyclosporine is a rapidly acting drug for the treatment of psoriasis and efficacious in the treatment of refractory disease, toxicity associated with prolonged use can be severe, which limits its use as maintenance therapy. We describe combination therapy of etanercept and low dose cyclosporine in the treatment of patients with psoriasis that was poorly controlled by other conventional agents.
The study sample consisted of 7 patients (n=7) 5 male and 2 female; age range 29~49 years, mean 37.7 years, previously treated with a variety of systemic agents, phototherapies and/or topical therapies for several years. In all cases, their psoriasis was only partially or not at all controlled by these treatments (Table 1). None of the 7 patients received conventional combination therapy, such as acitretin combined with cyclosporine or acitretin combined with methotrexate. Two patients in the study cohort had lower PASI score than the others (5.4, 9.6), because they did not response to conventional treatment and they wanted alternative treatment owing to reduced quality of life. They were included into the study. All 7 patients did not have other chronic illness.
At baseline, complete medical histories were taken and physical examinations were performed. Baseline laboratory tests included the following: red blood cell count, haemoglobin concentration, platelet count, markers of kidney and hepatic function (creatinine, urea, aspartate aminotransferase, alanine aminotransferase), triglyceride, total cholesterol, low density lipoprotein-cholesterol, high density lipoprotein-cholesterol, fasting plasma glucose, C-reactive protein, urinalysis and tests for antibodies against hepatitis B surface antigens and hepatitis C virus. The cohort also received tuberculosis skin testing and chest x-ray to exclude latent tuberculosis and other pulmonary infections. All 7 patients showed laboratory test results in the normal ranges and nonspecific lesions on the chest PA.
Etanercept was initiated at a conditioning dose of 50 mg weekly subcutaneous injections. Cyclosporine was added at an initial daily dose of 200 mg, given in 2 fractioned doses, until their lesions had almost disappeared. After a conditioning period, the dose of etanercept was reduced by 25~50 mg monthly and that of cyclosporine was reduced by 50~100 mg daily. Clinical response to combination therapy was measured by PASI score at every follow-up visit. Other safety analyses included vital signs and laboratory assessments were conducted every 4 weeks.
The 7 patients had a mean PASI score of 18.6 at baseline (Table 1). All patients underwent continuous combination treatment of etanercept (50 mg/week) and cyclosporine (200 mg/day) until their skin lesions almost disappeared, at which point the daily dose of cyclosporine was reduced by 100 mg/day and a subcutaneous injection of 25 mg etanercept was given bi-weekly. When the patients showed a stable skin condition with the reduced dose treatment, they were switched to maintenance therapy. The mean period of initial conditioning therapy was 6.85 weeks (Fig. 1). The initial mean PASI score was 18.6. After conditioning therapy, the mean PASI score was 0.94. Following maintenance therapy, it increased slightly to 1.25. The mean PASI reduction after conditioning therapy and maintenance therapy were 94.9% and 93.2%, respectively.
Combination therapy was generally well tolerated. There were no reports of severe adverse events during the treatment period, and no clinically significant changes were observed in clinical or laboratory values. T cell, B cell and NK cell counts of patients treated with this combination therapy for more than 1 year were checked to determine their immune status (Table 2), which revealed no specific finding.
Adverse events (AEs) included aggravation of tinea pedis and upper respiratory track infections, but a causal relationship between the combination therapy and AEs was unclear. Psoriasis was well controlled, and 5 patients continue to receive combination treatment up to the present time. One patient interrupted treatment due to financial pressures, and another discontinued therapy because of pregnancy.
The present report describes the clinical responses of 7 patients with moderate-to-severe psoriasis to a combination therapy of etanercept and low dose cyclosporine. Recently, dermatologists prefer etanercept and acitretin to treat psoriasis as a combination. Two patients of the study cohort were of childbearing age and other patients complained severe side effect of previous treatment by acitretin. Cyclosporine was therefore chosen as a combination agent.
A large, randomized, placebo-controlled study exclusively evaluated etanercept as a monotherapy for plaque psoriasis that enrolled 112 moderate-to severe psoriasis patients2. The mean PASI score in the study cohort was approximately 18. After 12 weeks of etanercept (25 mg, twice weekly) monotherapy, 30% of patients achieved a PASI of 75, while after 4 weeks of continuous therapy, 56% of patients achieved this score. In our study, after 6.85 weeks of combination therapy, 85% of patients achieved a PASI 90. This suggests that combination therapy led to quicker improvements and more extensive efficacy than etanercept monotherapy alone.
Lago et al.4 evaluated 30 patients with psoriasis treated with cyclosporine monotherapy for 8 weeks. Mean PASI before treatment was 26.32, and reduced to 3.71 after treatment.
In our study, after 6.85 weeks mean PASI was 0.94, showing slightly faster improvement when compared to cyclosporine monotherapy. We hypothesize that these therapeutic effects are due to the synergistic effects of combination therapy, as these 2 drugs have different therapeutic mechanisms. Cyclosporine acts on T cell cytokines, such as IL-1 while etanercept is a TNF-α inhibitor. These differing mechanisms lead to improved efficacy and at the same time minimized AEs, compared to higher doses of monotherapy of either agent. The combination requires reduced dosage of each agent, which is an important issue considering the cost of etanercept.
Each agent does have distinct side effects. The most significant side effects of cyclosporine are nephrotoxicity and hypertension5, while other side effects include infection, neoplasia, hyperlipidemia, hyperuricemia, hypercalemia and gastrointestinal problems. The most common side effect of etanercept is injection site reaction6. Other known side effects include infection, especially upper respiratory infections, tuberculosis reactivation and multiple sclerosis exacerbation. The nephrotoxicity of cyclosporine is both dose- and duration-dependent. According to the literature, lowering the daily dosage (<3 mg/kg) prevents nephrotoxicity7. Other studies have investigated renal function and structure in long-term low dose cyclosporine therapy in psoriasis patients and found only mild structural changes (1~2 mg/kg/day for 3.5 years)8. These side effects could be reduced by using low dose combination therapy. Both etanercept and infliximab have been used in combination with methotrexate in patients with rheumatoid arthritis. A previous study analyzed 100,000 patients with rheumatoid arthritis, many of whom were treated with other immunosuppressive agents such as corticosteroids or methotrexate that subsequently received treatment with etanercept or infliximab9. In this population, the incidence of infection was low. Costanzo et al.10 conducted a study in which cyclosporine was added at the time of relapse or aggravation during efalizumab therapy. The addition of 3.5~4.0 mg/kg/day of cyclosporine to efalizumab induced rapid clearance of relapsed plaques without significantly affecting clinical or laboratory parameters in all patients. No AEs were observed. This study also shows the effectiveness and safety of combination therapies using cyclosporine and a biologic agent. According to other small studies (a total of 6 patients), using a TNF-α inhibitor with cyclosporine caused no AEs11-14 and led to good responses (Table 3). However, with this combination therapy, additive immunosuppression must be considered, though in our study, no serious infections occurred.
Although cyclosporine has been associated with an increased incidence of lymphoproliferative disease in transplants patients under long term immunosuppression, no such correlation has been reported in the studies involving cyclosporine treated psoriasis9. This is most likely the result of lower treatment doses in psoriasis. Etanercept has not been associated with the development of lymphoproliferative diseases or malignancies, with the exception of 7 patients who developed squamous cell carcinoma after the introduction of etanercept in rheumatoid arthritis15. We are not in a position to exclude the potential of increased incidence of malignancy in this combination therapy. In order to validate the efficacy and safety of this combination therapy, more large-scale clinical studies are needed.
This study shows that combination therapy of etanercept and low dose cyclosporine is a possible new alternative treatment for refractory psoriasis. Combination therapies employing biologic and systemic agents are becoming more widely used in the treatment of psoriasis, as they treat symptoms more rapidly and effectively. In addition, this combination shows good cost effectiveness and accessibility. However, physicians must be aware of the possibility of infection, and we recommend combination treatment for patients without other chronic illnesses.
Figures and Tables
Fig. 1
Clinical photographs of patient 1, 5 and 6 at baseline (A, C, E), and 6 weeks after combination therapy (B), 2 weeks after combination therapy (D), 6 weeks after combination therapy (F), respectively.
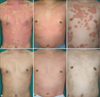
Table 1
Demographics, previous treatments, treatment characteristics of the patients studied, over the course of the study

References
1. Gudijonsson JE, Elder JT. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Psoriasis. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;169–198.
2. Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003. 139:1627–1632.

3. Stebbins WG, Lebwohl MG. Biologics in combination with nonbiologics: efficacy and safety. Dermatol Ther. 2004. 17:432–440.

4. Lago E, Carneiro S, Cuzzi T, Magalhaes G, Cassia F, Pessanha F, et al. Clinical and immunohistochemical assessment of the effect of cyclosporin in keratinocytes and dermal dendrocytes in psoriasis. J Cutan Pathol. 2007. 34:15–21.

6. Goldsmith DR, Wagstaff AJ. Etanercept: a review of its use in the management of plaque psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2005. 6:121–136.
7. Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, Jouanneau C, Jaudon MC, Maksud P, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol. 2002. 13:2962–2968.

8. Lowe NJ, Wieder JM, Rosenbach A, Johnson K, Kunkel R, Bainbridge C, et al. Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol. 1996. 35:710–719.

9. Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003. 49:S118–S124.

10. Costanzo A, Talamonti M, Spallone G, Botti E, Chimenti MS, Papoutsaki M, et al. Efficacy of short-term cyclosporine treatment to control psoriasis-related events during efalizumab therapy. Dermatology. 2009. 218:146–150.

11. Bellisai F, Giannitti C, Donvito A, Galeazzi M. Combination therapy with cyclosporine A and anti-TNF-alpha agents in the treatment of rheumatoid arthritis and concomitant hepatitis C virus infection. Clin Rheumatol. 2007. 26:1127–1129.

12. Iyer S, Yamauchi P, Lowe NJ. Etanercept for severe psoriasis and psoriatic arthritis: observations on combination therapy. Br J Dermatol. 2002. 146:118–121.

13. Gul U, Gonul M, Kilic A, Erdem R, Cakmak SK, Gunduz H. Treatment of psoriatic arthritis with etanercept, methotrexate, and cyclosporin A. Clin Ther. 2006. 28:251–254.

14. Javier P, Esteban D, Tatiana S, Amaro G. Cyclosporin A effectively controls recalcitrant psoriasis resistant to biologic therapy. J Am Acad Dermatol. 2007. 56:AB180.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download